Page 100 - AN-1-2
P. 100
Advanced Neurology Aging blood-brain barrier in stroke
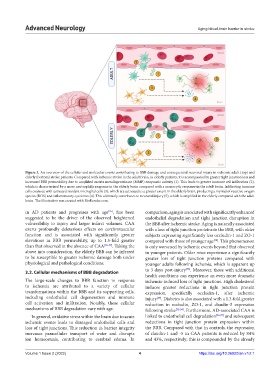
Figure 2. An overview of the cellular and molecular events contributing to BBB damage and consequential neuronal injury in ischemic adult (top) and
elderly (bottom) stroke patients. Compared with ischemic stroke in the adult brain, in elderly patients, it is accompanied by greater tight junction loss and
increased BBB permeability due to amplified matrix metalloproteinase (MMP) enzymatic activity (1). This leads to greater immune cell infiltration (2),
which is characterized by a more neutrophilic response in the elderly brain compared with a monocytic response in the adult brain. Infiltrating immune
cells coalesce with activated resident microglial cells (3), which are activated to a greater extent in the elderly brain, producing a myriad of reactive oxygen
species (ROS) and inflammatory cytokines (4). This ultimately contributes to neuronal injury (5), which is amplified in the elderly compared with the adult
brain. The illustration was created with BioRender.com.
in AD patients and progresses with age , has been comparison, aging is associated with significantly enhanced
[35]
suggested to be the driver of the observed heightened endothelial degradation and tight junction disruption in
vulnerability to injury and larger infarct volumes. CAA the BBB after ischemic stroke. Aging is naturally associated
exerts profoundly deleterious effects on cerebrovascular with a loss of tight junction proteins in the BBB, with older
function and is associated with significantly greater subjects expressing significantly less occludin-1 and ZO-1
elevations in BBB permeability, up to 1.5-fold greater compared with those of younger age . This phenomenon
[30]
than that observed in the absence of CAA [35-37] . Taking the is only worsened by ischemic events beyond that observed
above into consideration, the elderly BBB can be inferred in younger patients. Older mice experience a significantly
to be susceptible to greater ischemic damage both under greater loss of tight junction proteins compared with
physiological and pathological conditions. younger adults following ischemia, which is apparent up
[38]
3.2. Cellular mechanisms of BBB degradation to 3 days post-injury . Moreover, those with additional
health conditions can experience an even more dramatic
The large-scale changes to BBB function in response ischemia-induced loss of tight junctions. High cholesterol
to ischemia are attributed to a variety of cellular induces greater reductions in tight junction protein
transformations within the BBB and its supporting cells, expression, specifically occludin-1, after ischemic
including endothelial cell degeneration and immune injury . Diabetes is also associated with a 1.3-fold greater
[39]
cell activation and infiltration. Notably, these cellular reduction in occludin, ZO-1, and claudin-5 expression
mechanisms of BBB degradation vary with age. following stroke [32-34] . Furthermore, AD-associated CAA is
In general, oxidative stress within the brain due to acute linked to endothelial cell degradation [36,37] and subsequent
ischemic events leads to damaged endothelial cells and reductions in tight junction protein expression within
loss of tight junctions. This reduction in barrier integrity the BBB. Compared with that in controls, the expression
increases paracellular transport of water and disrupts of claudin-1 and -5 in CAA patients is reduced by 84%
ion homeostasis, contributing to cerebral edema. In and 43%, respectively; this is compounded by the already
Volume 1 Issue 2 (2022) 4 https://doi.org/10.36922/an.v1i2.1