Page 30 - AN-3-4
P. 30
Advanced Neurology Anticoagulants as neuroprotective therapeutics
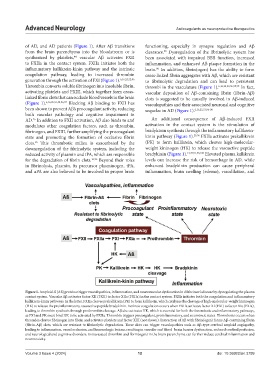
of AD, and AD patients (Figure 1). After Aβ transitions functioning, especially in synapse regulation and Aβ
from the brain parenchyma into the bloodstream or is clearance. Dysregulation of the fibrinolytic system has
96
synthesized by platelets, vascular Aβ activates FXII been associated with impaired BBB function, increased
44
to FXIIa in the contact system. FXIIa initiates both the inflammation, and enhanced Aβ plaque formation in the
inflammatory kallikrein-kinin pathway and the intrinsic brain. In addition, fibrin(ogen) has the ability to form
96
coagulation pathway, leading to increased thrombin cross-linked fibrin aggregates with Aβ, which are resistant
generation through the activation of FXI (Figure 1). 6,16,25,52,96 to fibrinolytic degradation and can lead to persistent
Thrombin converts soluble fibrinogen into insoluble fibrin, thrombi in the vasculature (Figure 1). 6,16,25,53,96,98,99 In fact,
activating platelets and FXIII, which together form cross- vascular deposition of Aβ-containing fibrin (fibrin-Aβ)
linked fibrin clots that can occlude blood vessels in the brain clots is suggested to be causally involved in Aβ-induced
(Figure 1). 6,16,25,51,52,96,97 Blocking Aβ binding to FXII has vasculopathies and their associated neuronal and cognitive
been shown to prevent Aβ’s procoagulant activity, reducing sequelae in AD (Figure 1). 6,16,25,53,96-98
both vascular pathology and cognitive impairment in
AD. In addition to FXII activation, Aβ also binds to and An additional consequence of Aβ-induced FXII
25
modulates other coagulation factors, such as thrombin, activation in the contact system is the stimulation of
fibrinogen, and FXIII, further amplifying the procoagulant bradykinin synthesis through the inflammatory kallikrein-
state and promoting the formation of occlusive fibrin kinin pathway (Figure 1). 25,96 FXIIa activates prekallikrein
clots. This thrombotic milieu is exacerbated by the (PK) to form kallikrein, which cleaves high-molecular-
16
downregulation of the fibrinolytic system, including the weight kininogen (HK) to release the vasoactive peptide
reduced activity of plasmin and tPA, which are responsible bradykinin (Figure 1). 14,25,51,96,100 Elevated plasma kallikrein
for the degradation of fibrin clots. 16,96 Beyond their roles levels can increase the risk of hemorrhage in AD, while
in fibrinolysis, plasmin, its precursor plasminogen, tPA, enhanced bradykinin production can cause peripheral
and uPA are also believed to be involved in proper brain inflammation, brain swelling (edema), vasodilation, and
Figure 1. Amyloid-ß (Aß) proteins trigger vasculopathies, inflammation, and neurovascular dysfunction in Alzheimer’s disease by dysregulating the plasma
contact system. Vascular Aβ activates factor XII (FXII) to factor XIIa (FXIIa) in the contact system. FXIIa initiates both the coagulation and inflammatory
kallikrein-kinin pathways. In the latter, FXIIa cleaves prekallikrein (PK) to form kallikrein, which catalyzes the cleavage of high-molecular-weight kininogen
(HK) to release the proinflammatory, vasoactive peptide bradykinin. Intrinsic coagulation occurs when FXIIa activates factor XI (FXI) to factor XIa (FXIa),
leading to thrombin synthesis through prothrombin cleavage. Aβ also activates HK, which is essential for both the thrombotic and inflammatory pathways,
as FXI and PK must bind HK to be activated by FXIIa. Thrombin triggers procoagulant, proinflammatory, and neurotoxic states. Thrombosis occurs when
thrombin cleaves fibrinogen into fibrin and activates platelets and factor XIII (not shown). Interaction of Aβ with fibrin(ogen) forms Aβ-containing fibrin
(fibrin-Aβ) clots, which are resistant to fibrinolytic degradation. These clots can trigger vasculopathies such as Aβ-type cerebral amyloid angiopathy,
leading to inflammation, vessel occlusion, and hemorrhagic lesions, resulting in vascular and blood–brain barrier dysfunction, reduced cerebral perfusion,
and neurological and cognitive disorders. Extravasated thrombin and fibrin(ogen) in the brain parenchyma can further induce cerebral inflammation and
neurotoxicity.
Volume 3 Issue 4 (2024) 12 doi: 10.36922/an.3799