Page 80 - GPD-4-1
P. 80
Gene & Protein in Disease Sickle cell disease’s journey
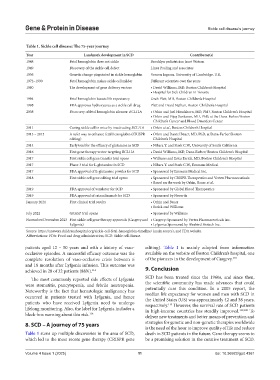
Table 1. Sickle cell disease: The 75-year journey
Year Landmark development in SCD Contributor(s)
1948 Fetal hemoglobin does not sickle Brooklyn pediatrician Janet Watson
1949 Discovery of the sickle cell defect Linus Pauling and associates
1956 Genetic change pinpointed in sickle hemoglobin Vernon Ingram, University of Cambridge, U.K.
1972–1990 Fetal hemoglobin makes sickle cell milder Different scientists over the years
1980 The development of gene delivery vectors • David Williams, MD, Boston Children’s Hospital
• Hospital for Sick Children in Toronto
1994 Fetal hemoglobin boosts life expectancy Orah Platt, MD, Boston Children’s Hospital
1998 FDA approves hydroxyurea as a sickle cell drug Platt and David Nathan, Boston Children’s Hospital
2008 Discovery of fetal hemoglobin silencer: BCL11A • Orkin and Joel Hirschhorn, MD, PhD, Boston Children’s Hospital
• Orkin and Vijay Sankaran, MD, PhD, at the Dana-Farber/Boston
Children’s Cancer and Blood Disorders Center
2011 Curing sickle cell in mice by inactivating BCL11A • Orkin et al., Boston Children’s Hospital
2013 – 2015 A safer way to enhance fetal hemoglobin (CRISPR • Orkin and Daniel Bauer, MD, PhD, at Dana-Farber/Boston
editing) Children’s Hospital
2014 Early trial for the efficacy of glutamine in SCD • Nihara Y. and Stark C.W., University of South California
2016 First gene therapy vector targeting BCL11A • David Williams, MD, Dana-Farber/ Boston Children’s Hospital
2017 First sickle cell gene transfer trial opens • Williams and Erica Esrick, MD, Boston Children’s Hospital
2017 Phase 3 trial for L-glutamine in SCD • Nihara Y. and Stark C.W., Emmaus Medical
2017 FDA approval of L-glutamine powder for SCD • Sponsored by Emmaus Medical Inc.
2018 First sickle cell gene editing trial opens • Sponsored by CRISPR Therapeutics and Vertex Pharmaceuticals
• Based on the work by Orkin, Bauer et al.
2019 FDA approval of voxelotor for SCD • Sponsored by Global Blood Therapeutics
2019 FDA approval of crizanlizumab for SCD • Sponsored by Novartis
January 2021 First clinical trial results • Orkin and Bauer
• Esrick and Williams
July 2022 GRASP trial opens • Sponsored by Williams
November/December 2023 First sickle cell gene therapy approvals (Casgevy and • Casgevy: Sponsored by Vertex Pharmaceuticals Inc.
Lyfgenia) • Lyfgenia: Sponsored by Bluebird Biotech Inc.
Source: https://answers.childrenshospital.org/sickle-cell-fetal-hemoglobin-timeline/ (main source), and FDA website.
Abbreviations: FDA: Food and drug administration; SCD: Sickle cell disease.
patients aged 12 – 50 years and with a history of vaso- editing). Table 1 is mainly adopted from information
occlusive episodes. A successful efficacy outcome was the available on the website of Boston Children’s hospital, one
complete resolution of vaso-occlusive crisis between 6 of the pioneers in the development of Casgevy. 118
and 18 months after Lyfgenia infusion. This outcome was
achieved in 28 of 32 patients (88%). 114 9. Conclusion
The most commonly reported side effects of Lyfgenia SCD has been treated since the 1960s, and since then,
were stomatitis, pancytopenia, and febrile neutropenia. the scientific community has made advances that could
Noteworthy is the fact that hematologic malignancy has potentially cure this condition. In a 2005 report, the
median life expectancy for women and men with SCD in
occurred in patients treated with Lyfgenia, and hence the United States (US) was approximately 42 and 38 years,
patients who have received Lyfgenia need to undergo respectively. However, the survival rate of SCD patients
119
lifelong monitoring. Also, the label for Lyfgenia includes a in high-income countries has steadily improved. 119,120 To
black-box warning about this risk. 114 deliver new treatments and better means of prevention and
8. SCD – A journey of 75 years strategies for genetic and non-genetic therapies worldwide
is the need of the hour to improve quality of life and reduce
Table 1 sums up multiple discoveries in the area of SCD, death in SCD patients in the future. Gene therapy seems to
which led to the most recent gene therapy (CRISPR gene be a promising solution in the curative treatment of SCD.
Volume 4 Issue 1 (2025) 11 doi: 10.36922/gpd.4361