Page 40 - ITPS-7-4
P. 40
INNOSC Theranostics and
Pharmacological Sciences Pediatric drug regulations in India
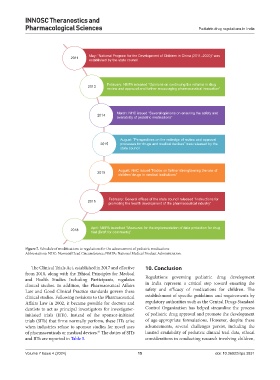
May: “National Program for the Development of Children in China (2011–2020)” was
2011 established by the state council
February: NMPA released “Opinions on continuing the reforms in drug
2013 review and approval and further encouraging pharmaceutical innovation”
March: NHC issued “Several opinions on ensuring the safety and
2014
availability of pediatric medications”
August: “Perspectives on the redesign of review and approval
2015 processes for drugs and medical devices” was released by the
state council
August: NHC issued “Notice on further strengthening the use of
2015
children’ drugs in medical institutions”
2016 February: General offices of the state council released “Instructions for
promoting the health development of the pharmaceutical industry”
2018 April: NMPA launched “Measures for the implementation of data protection for drug
trial (Draft for comments)”
Figure 7. Schedule of modifications to regulations for the advancement of pediatric medications
Abbreviations: NHC: Neonatal Head Circumference; NMPA: National Medical Product Administration.
The Clinical Trials Act, established in 2017 and effective 10. Conclusion
from 2018, along with the Ethical Principles for Medical
and Health Studies Including Participants, regulates Regulations governing pediatric drug development
clinical studies. In addition, the Pharmaceutical Affairs in India represent a critical step toward ensuring the
Law and Good Clinical Practice standards govern these safety and efficacy of medications for children. The
clinical studies. Following revisions to the Pharmaceutical establishment of specific guidelines and requirements by
Affairs Law in 2002, it became possible for doctors and regulatory authorities such as the Central Drugs Standard
dentists to act as principal investigators for investigator- Control Organization has helped streamline the process
initiated trials (IITs). Instead of the sponsor-initiated of pediatric drug approval and promote the development
trials (SITs) that firms normally perform, these IITs arise of age-appropriate formulations. However, despite these
when industries refuse to sponsor studies for novel uses advancements, several challenges persist, including the
of pharmaceuticals or medical devices. The duties of SITs limited availability of pediatric clinical trial data, ethical
61
and IITs are reported in Table 5. considerations in conducting research involving children,
Volume 7 Issue 4 (2024) 15 doi: 10.36922/itps.3831