Page 13 - MI-2-1
P. 13
Microbes & Immunity Sepsis and gut microbiome
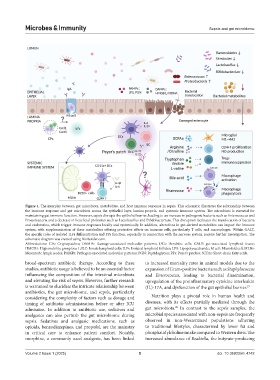
Figure 1. The interplay between gut microbiota, metabolites, and host immune response in sepsis. This schematic illustrates the relationship between
the immune response and gut microbiota across the epithelial layer, lamina propria, and systemic immune system. The microbiota is essential for
maintaining gut immune function. However, sepsis disrupts the epithelial barrier, leading to an increase in pathogenic bacteria such as Enterococcus and
Proteobacteria and a decrease in beneficial probiotics such as Lactobacillus and Bifidobacterium. This disruption facilitates the translocation of bacteria
and endotoxins, which trigger immune responses locally and systemically. In addition, alterations in gut-derived metabolites can impact the immune
system, with supplementation of these metabolites offering protective effects on immune cells, particularly T cells, and macrophages. Within GALT,
the specific roles of isolated ILFs differentiation and PPs function, especially in connection with the nervous system, require further investigation. The
schematic diagram was created using BioRender.com.
Abbreviations: CPs: Cryptopatches; DAMPs: Damage-associated molecular patterns; DCs: Dendritic cells; GALT: gut-associated lymphoid tissue;
HMGB1: High mobility group box 1; ILC: Innate lymphoid cells; ILFs: Isolated lymphoid follicles; LPS: Lipopolysaccharide; M cell: Microfold cell; MLNs:
Mesenteric lymph nodes; PAMPs: Pathogen-associated molecular patterns; PGN: Peptidoglycan; PPs: Peyer’s patches; SCFAs: Short-chain fatty acids.
broad-spectrum antibiotic therapy. According to these to increased mortality rates in animal models due to the
studies, antibiotic usage is believed to be an essential factor expansion of Gram-positive bacteria such as Staphylococcus
influencing the composition of the intestinal microbiota and Enterococcus, leading to bacterial dissemination,
and elevating the risk of sepsis. However, further research upregulation of the proinflammatory cytokine interleukin
is warranted to elucidate the intricate relationship between (IL)-17A, and dysfunction of the gut epithelial barrier. 27
antibiotics, the gut microbiome, and sepsis, particularly
considering the complexity of factors such as dosage and Nutrition plays a pivotal role in human health and
timing of antibiotic administration before or after ICU diseases, with its effects partially mediated through the
28
admission. In addition to antibiotic use, sedatives and gut microbiota. In contrast to the sepsis samples, the
analgesics can also perturb the gut microbiome during microbial species associated with non-sepsis are frequently
sepsis. Sedatives and analgesic medications, such as observed in non-Westernized populations adhering
opioids, benzodiazepines, and propofol, are the mainstay to traditional lifestyles, characterized by lower fat and
in critical care to enhance patient comfort. Notably, phosphatidylcholine intake compared to Western diets. The
morphine, a commonly used analgesic, has been linked increased abundance of Ezakiella, the butyrate-producing
Volume 2 Issue 1 (2025) 5 doi: 10.36922/mi.4742