Page 47 - AN-2-1
P. 47
Advanced Neurology Piribedil for Parkinson’s disease
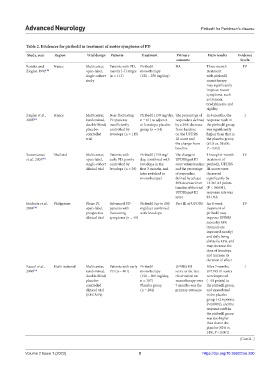
Table 2. Evidences for piribedil in treatment of motor symptoms of PD
Study, year Region Trial design Patients Treatment Primary Main results Evidence
outcome levela
Rondot and France Multicenter, Patients with PD, Piribedil NA Three-month IV
Ziegler, 1992 [40] open-label, mainly I–II stages monotherapy treatment
single-cohort (n = 113) (150 – 250 mg/day) with piribedil
study monotherapy
may significantly
improve motor
symptoms, such
as tremors,
bradykinesia, and
rigidity
Ziegler et al., France Multicenter, Non-fluctuating Piribedil (150 mg/day, The percentage of In 6 months, the I
2003 [42] randomized, PD patients n = 61) as adjunct responders defined response rateb in
double-blind, insufficiently to levodopa placebo by a 30% decrease the piribedil group
placebo- controlled by group (n = 54) from baseline was significantly
controlled levodopa (n = 115) on the UPDRS higher than that in
trial III score and the placebo group
the change from (61.8 vs. 39.6%;
baseline P = 0.02)
Suwantamee Thailand Multicenter, Patients with Piribedil (150 mg/ The change in Through 6-month IV
et al., 2004 [41] open-label, early PD, poorly day, combined with UPDRS part III treatment of
single-cohort controlled by levodopa in the score versus baseline piribedil, UPDRS
clinical trial levodopa (n = 29) first 3 months, and and the percentage III scores were
later switched to of responders decreased
monotherapy) defined by at least significantly by
30% decrease from 13.3±10.3 points
baseline of the total (P < 0.0001),
UPDRS part III response rate was
score 93.1%b
Evidente et al., Philippines Phase IV, Advanced PD Piribedil (up to 150 Part III of UPDRS An 8-week IV
2004 [36] open-label, patients with mg/day) combined treatment of
prospective fluctuating with levodopa piribedil may
clinical trial symptoms (n = 49) improve UPDRS
scores by 48%
(tremors are
improved mostly)
and daily living
ability by 43%, and
may decrease the
dose of levodopa
and increase its
duration of effect
Rascol et al., Multi-national Multicenter, Patients with early Piribedil UPDRS III After 7 months, I
2006 [39] randomized, PD (n = 401) monotherapy score as the last UPDRS III scores
double-blind, (150 – 300 mg/day, observation on were improved
placebo- n = 197) monotherapy over (−4.9 points) in
controlled Placebo group 7 months was the the piribedil group,
clinical trial (n = 204) primary outcome and exacerbated
(REGAIN) in the placebo
group (+2.6 points;
P<0.0001), and the
response rateb in
the piribedil group
was also higher
than that in the
placebo (42% vs.
14%, P < 0.001)
(Cont’d...)
Volume 2 Issue 1 (2023) 5 https://doi.org/10.36922/an.290