Page 24 - AN-4-2
P. 24
Advanced Neurology SARS-CoV-2 mechanisms of neurological impact
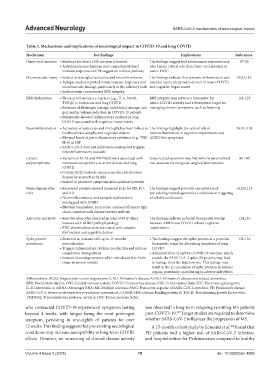
Table 3. Mechanisms and implications of neurological impact in COVID‑19 and long COVID
Mechanism Key findings Implications References
Direct viral invasion • Evidence for direct CNS invasion is limited. The findings suggest that autoimmune responses may 87-89
• Autoimmune mechanisms and compartmentalized play a more critical role than direct viral invasion in
immune responses in CSF suggest an indirect pathway neuro-PASC.
Microvascular injury • Linked to microglial activation and microthrombosis The findings indicate that systemic inflammation and 50,91,119
• Autopsy studies reported innate immune responses and vascular injury are primary drivers of neuro‑COVID
microvascular damage, particularly in the olfactory bulb and cognitive impairments
• Spike protein compromises BBB integrity.
BBB dysfunction • Elevated inflammatory markers (e.g., IL‑6, S100β, BBB integrity may serve as a biomarker for 121-123
TGF-β) in both acute and long COVID neuro-COVID severity and a therapeutic target for
• Evidence of fibrinogen leakage, endothelial damage, and managing chronic symptoms, such as brain fog
gray matter volume reduction in COVID-19 patients
• Persistently elevated inflammatory markers in long
COVID associated with cognitive impairments
Neuroinflammation • Activation of astrocytes and microglia has been linked to The findings highlight the central role of 24,101,103
localized brain atrophy and cognitive deficits neuroinflammation in cognitive impairments and
• Elevated levels of pro‑inflammatory cytokines (e.g., TNF, ADRD-like symptoms
IL-6) in CSF
• SARS‑CoV‑2 does not replicate in neurons but triggers
neuroinflammatory cascades
Genetic • Variants of ACE2 and TMPRSS2 were associated with Genetic predisposition may help inform personalized 98-100
polymorphisms increased susceptibility to severe disease and long risk assessments and guide targeted interventions
COVID
• Certain ACE2 variants can increase the risk of severe
disease by as much as 28-fold
• Linked to persistent symptoms in hospitalized patients
Neurodegenerative • Recovered patients showed increased risks for MS, PD, The findings suggest potential exacerbation of 14,105,113
risks and AD pre-existing neurodegenerative conditions or triggering
• Neuroinflammatory and synaptic dysfunctions of subclinical diseases
overlapped with ADRD
• Elevated biomarkers, such as tau and neurofilament light
chain, correlate with disease severity and risk
Astrocyte reactivity • Reactive astrocytes observed in long COVID share The findings indicate potential therapeutic overlap 124,125
features with ADRD pathophysiology between ADRD and COVID-related cognitive
• EEG abnormalities were associated with synaptic impairments
dysfunction and cognitive decline
Spike protein • Detected in immune cells up to 15 months • The findings suggest the spike protein as a potential 131-133
persistence post-infection therapeutic target for alleviating symptoms of long
• Triggers inflammatory cytokine production and induces COVID
coagulation dysregulation • Administration of mRNA COVID‑19 vaccines, which
• Induces neurodegeneration when introduced into brain encode the SARS-CoV-2 spike (S) protein, may lead
tissue in animal models to leakage from the injection site. This leakage may
result in the accumulation of spike proteins in various
organs, potentially contributing to adverse side effects
Abbreviations: ACE2: Angiotensin‑converting enzyme 2; AD: Alzheimer’s disease; ADRD: Alzheimer’s disease and related dementias;
BBB: Blood‑brain barrier; CNS: Central nervous system; COVID: Coronavirus disease; CSF: Cerebrospinal fluid; EEG: Electroencephalogram;
IL‑6: Interleukin‑6; mRNA: Messenger RNA; MS: Multiple sclerosis; PASC: Post‑acute sequelae of SARS‑CoV‑2 infection; PD: Parkinson’s disease;
SARS‑CoV‑2: Severe acute respiratory syndrome coronavirus 2; S100β: S100 calcium‑binding protein B; TGF‑β: Transforming growth factor‑beta;
TMPRSS2: Transmembrane protease, serine 2; TNF: Tumor necrosis factor.
who contracted COVID-19 experienced symptoms lasting was observed in long-term relapsing-remitting MS patients
107
beyond 4 weeks, with fatigue being the most prolonged post-COVID-19. Larger studies are required to determine
symptom, persisting in one-eighth of patients for over whether SARS-CoV-2 influences the progression of MS.
12 weeks. This finding suggests that pre-existing neurological A 15-month cohort study by Zenesini et al. found that
108
conditions may increase susceptibility to long-term COVID PD patients had a higher risk of SARS-CoV-2 infection
effects. However, no worsening of clinical disease activity and hospitalization for Parkinsonism compared to healthy
Volume 4 Issue 2 (2025) 18 doi: 10.36922/an.4909