Page 85 - ARNM-3-2
P. 85
Advances in Radiotherapy
& Nuclear Medicine Radiotherapy in node-positive bladder cancer
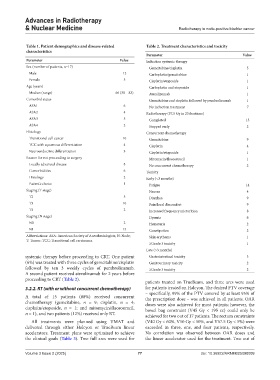
Table 1. Patient demographics and disease‑related Table 2. Treatment characteristics and toxicity
characteristics
Parameter Value
Parameter Value Induction systemic therapy
Sex (number of patients, n=17) Gemcitabine/cisplatin 5
Male 12 Carboplatin/gemcitabine 1
Female 5 Cisplatin/etoposide 1
Age (years) Carboplatin and etoposide 1
Median (range) 66 (30 – 83) Atezolizumab 1
Comorbid status Gemcitabine and cisplatin followed by pembrolizumab 1
ASA1 6 No induction treatment 7
ASA2 4 Radiotherapy (57.5 Gy in 23 fractions)
ASA3 5 Completed 15
ASA4 2 Stopped early 2
Histology Concurrent chemotherapy
Transitional cell cancer 10 Gemcitabine 9
TCC with squamous differentiation 4 Cisplatin 4
Neuroendocrine differentiation 3 Cisplatin/etoposide 1
Reason for not proceeding to surgery Mitomycin/fluorouracil 1
Locally advanced disease 8 No concurrent chemotherapy 2
Comorbidities 6 Toxicity
Histology 2 Early (<3 months)
Patient’s choice 1 Fatigue 14
Staging (T stage) Nausea 4
T2 5 Diarrhea 9
T3 10 Pain/local discomfort 9
T4 2 Increased frequency micturition 8
Staging (N stage) Dysuria 6
N0 2 Hematuria 2
N1 15 Constipation 2
Abbreviations: ASA: American Society of Anesthesiologists; N: Node; Skin erythema 1
T: Tumor; TCC: Transitional cell carcinoma.
≥Grade 3 toxicity 1
Late (>3 months)
systemic therapy before proceeding to CRT. One patient Gastrointestinal toxicity 3
(6%) was treated with three cycles of gemcitabine/cisplatin Genitourinary toxicity 2
followed by ten 3 weekly cycles of pembrolizumab. ≥Grade 3 toxicity 2
A second patient received atezolizumab for 2 years before
proceeding to CRT (Table 2).
patients treated on TrueBeam, and three arcs were used
3.2.2. RT (with or without concurrent chemotherapy) for patients treated on Halcyon. The desired PTV coverage
– specifically, 99% of the PTV covered by at least 95% of
A total of 15 patients (88%) received concurrent the prescription dose – was achieved in all patients. OAR
chemotherapy (gemcitabine, n = 9; cisplatin, n = 4; doses were also achieved for most patients; however, the
cisplatin/etoposide, n = 1; and mitomycin/fluorouracil, bowel bag constraint (V45 Gy < 195 cc) could only be
n = 1), and two patients (12%) received only RT. achieved for two out of 17 patients. The rectum constraints
All treatments were planned using VMAT and (V40 Gy < 60%, V50 Gy < 50%, and V57.5 Gy < 5%) were
delivered through either Halcyon or TrueBeam linear exceeded in three, one, and four patients, respectively.
accelerators. Treatment plans were optimized to achieve No correlation was observed between OAR doses and
the clinical goals (Table 3). Two full arcs were used for the linear accelerator used for the treatment. Two out of
Volume 3 Issue 2 (2025) 77 doi: 10.36922/ARNM025090009