Page 28 - GTM-4-2
P. 28
Global Translational Medicine Small RNA therapy for pancreatic cancer
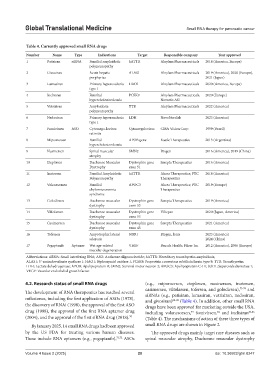
Table 4. Currently approved small RNA drugs
Number Name Type Indications Target Responsible company Year approved
1 Patisiran siRNA Familial amyloidotic hATTR Alnylam Pharmaceuticals 2018 (America, Europe)
polyneuropathy
2 Givosiran Acute hepatic ALAS1 Alnylam Pharmaceuticals 2019 (America), 2020 (Europe),
porphyrias 2021 (Japan)
3 Lumasiran Primary hyperoxaluria HAO1 Alnylam Pharmaceuticals 2020 (America, Europe)
type 1
4 Inclisiran Familial PCSK9 Alnylam Pharmaceuticals, 2020 (Europe)
hypercholesterolemia Novartis AG
5 Vutrisiran Amyloidosis TTR Alnylam Pharmaceuticals 2022 (America)
polyneuropathy
6 Nedosiran Primary hyperoxaluria LDH NovoNordisk 2023 (America)
type 1
7 Fomivirsen ASO Cytomega-lovirus Cytomegalovirus CIBA Vision Corp 1999 (Brazil)
retinitis
8 Mipomersen Familial APOB gene Kastle Therapeutics 2013 (Argentina)
hypercholesterolemia
9 Nusinersen Spinal muscular SMN2 Biogen 2016 (America), 2019 (China)
atrophy
10 Eteplirsen Duchenne Muscular Dystrophin gene Sarepta Therapeutics 2016 (America)
Dystrophy exon 51
11 Inotersen Familial Amyloidotic hATTR Akcea Therapeutics; PTC 2018 (America)
Polyneuropathy Therapeutics
12 Volanesorsen Familial APOC3 Akcea Therapeutics; PTC 2019 (Europe)
chylomicronemia Therapeutics
syndrome
13 Golodirsen Duchenne muscular Dystrophin gene Sarepta Therapeutics 2019 (America)
dystrophy exon 53
14 Viltolarsen Duchenne muscular Dystrophin gene Viltepso 2020 (Japan, America)
dystrophy exon 53
15 Casimersen Duchenne muscular Dystrophin gene Sarepta Therapeutics 2021 (America)
dystrophy exon 45
16 Tofersen Amyotrophic lateral SOD1 Biogen, lonis 2023 (America)
sclerosis 2024 (China)
17 Pegaptanib Aptamer Wet age-related VEGF Bausch Health; Pfizer lnc 2012 (America), 2006 (Europe)
macular degeneration
Abbreviations: siRNA: Small interfering RNA; ASO: Antisense oligonucleotide; hATTR: Hereditary transthyretin amyloidosis;
ALAS1: 5’-aminolevulinate synthase 1; HAO1: Hydroxyacid oxidase 1; PCSK9: Proprotein convertase subtilisin/kexin type 9; TTR: Transthyretin;
LDH: Lactate dehydrogenase; APOB: Apolipoprotein B; SMN2: Survival motor neuron 2; APOC3: Apolipoprotein C-III; SOD1: Superoxide dismutase 1;
VEGF: Vascular endothelial growth factor.
4.2. Research status of small RNA drugs (e.g., mipomersen, eteplirsen, nusinersen, inotersen,
casimersen, viltolarsen, tofersen, and golodirsen), 73-79 and
The development of RNA therapeutics has reached several siRNAs (e.g., patisiran, lumasiran, vutrisiran, nedosiran,
milestones, including the first application of ASOs (1978), and givosiran) 80-83 (Table 4). In addition, other small RNA
the discovery of RNAi (1998), the approval of the first ASO drugs have been approved for marketing outside the USA,
drug (1998), the approval of the first RNA aptamer drug including volanesorsen, fomivirsen, and inclisiran 85,86
84
79
(2004), and the approval of the first siRNA drug (2018). 70 (Table 4). The mechanisms of action of these three types of
By January 2025, 14 small RNA drugs had been approved small RNA drugs are shown in Figure 2.
by the US FDA for treating various human diseases. The approved drugs mainly target rare diseases such as
These include RNA aptamers (e.g., pegaptanib), 71,72 ASOs spinal muscular atrophy, Duchenne muscular dystrophy
Volume 4 Issue 2 (2025) 20 doi: 10.36922/gtm.8247