Page 30 - GTM-4-2
P. 30
Global Translational Medicine Small RNA therapy for pancreatic cancer
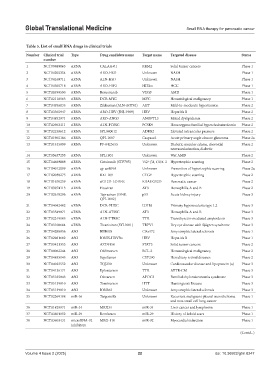
Table 5. List of small RNA drugs in clinical trials
Number Clinical trial Type Drug candidate name Target name Targeted disease Status
number
1 NCT00689065 siRNA CALAA-01 RRM2 Solid tumor cancers Phase 1
2 NCT04202354 siRNA ARO-HSD Unknown NASH Phase 1
3 NCT04169711 siRNA ALN-HSD Unknown NASH Phase 1
4 NCT04565718 siRNA ARO-HIF2 HIF2α HCC Phase 1
5 NCT00499590 siRNA Bevasiranib VEGF AMD Phase 1
6 NCT02110563 siRNA DCR-MYC MYC Hematological malignancy Phase 1
7 NCT04936035 siRNA Zilebesiran (ALN-AGT01) AGT Mild-to-moderate hypertension Phase 2
8 NCT03365947 siRNA ARO-HBV (JNJ-3989) HBV Hepatitis B Phase 2
9 NCT04832971 siRNA ARO-ANG3 ANGPTL3 Mixed dyslipidemia Phase 2
10 NCT02963311 siRNA ALN-PCSSC PCSK9 Homozygous familial hypercholesterolemia Phase 2
11 NCT02250612 siRNA SYL040012 ADRB2 Elevated intraocular pressure Phase 2
12 NCT01965106 siRNA QPI-1007 Caspase2 Acute primary angle closure glaucoma Phase 2a
13 NCT01445899 siRNA PF-0423655 Unknown Diabetic macular edema, choroidal Phase 2
neovascularization, diabetic
14 NCT05637255 siRNA SYL1801 Unknown Wet AMD Phase 2
15 NCT04669808 siRNA Cotsiranib (STP705) TGF-β1, COX-2 Hypertrophic scarring Phase 2
16 NCT04012099 siRNA cp-asiRNA Unknown Prevention of hypertrophic scarring Phase 2a
17 NCT02030275 siRNA RXI-109 CTGF Hypertrophic scarring Phase 2
18 NCT01676259 siRNA siG12D-LODER KRAS G12D Pancreatic cancer Phase 2
19 NCT03974113 siRNA Fitusiran AT3 Hemophilia A and B Phase 2
20 NCT02610296 siRNA Teprasiran (I5NP, p53 Acute kidney injury Phase 3
QPI-1002)
21 NCT04042402 siRNA DCR-PHXC LDHA Primary hyperoxaluria type 1,2 Phase 3
22 NCT03549871 siRNA ALN-AT3SC AT3 Hemophilia A and B Phase 3
23 NCT02319005 siRNA ALN-TTRSC TTR Transthyretin-mediated amyloidosis Phase 3
24 NCT03108664 siRNA Tivanisiran (SYL1001) TRPV1 Dry eye disease with Sjögren syndrome Phase 3
25 NCT04288856 ASO BIIB078 C9orf72 Amyotrophic lateral sclerosis Phase 1
26 NCT02981602 ASO IONIS-HBVRx HBV Hepatitis B Phase 1
27 NCT03421353 ASO AZD9150 STAT3 Solid tumor cancers Phase 2
28 NCT00062244 ASO Oblimersen BCL-2 Hematological malignancy Phase 2
29 NCT04855045 ASO Sepofarsen CEP290 Hereditary retinal diseases Phase 2
30 NCT04023552 ASO TQJ230 Unknown Cardiovascular disease and lipoprotein (a) Phase 3
31 NCT04136171 ASO Eplontersen TTR ATTR-CM Phase 3
32 NCT05185843 ASO Olezarsen APOC3 Familial chylomicronemia syndrome Phase 3
33 NCT05139810 ASO Tominersen HTT Huntington’s Disease Phase 3
34 NCT05139810 ASO ION363 Unknown Amyotrophic lateral sclerosis Phase 3
35 NCT02369198 miR-16 TargomiRs Unknown Recurrent malignant pleural mesothelioma Phase 1
and non-small cell lung cancer
36 NCT01829971 miR-34 MRX34 miR-34 Liver cancer and lymphoma Phase 1
37 NCT03601052 miR-29 Remlarsen miR-29 History of keloid scars Phase 1
38 NCT03603431 microRNA-92 MRG-110 miR-92 Myocardial infarction Phase 1
inhibitors
(Contd...)
Volume 4 Issue 2 (2025) 22 doi: 10.36922/gtm.8247