Page 580 - IJB-10-4
P. 580
International Journal of Bioprinting 3D-printing silicone patient-specific soft-tissue expander
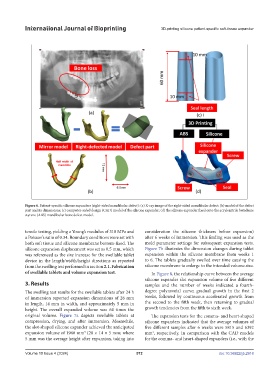
Figure 6. Patient-specific silicone expanders (right-sided mandibular defect): (a) X-ray image of the right-sided mandibular defect; (b) model of the defect
part and its dimensions; (c) computer-aided design (CAD) model of the silicone expander; (d) the silicone expander fixed onto the acrylonitrile butadiene
styrene (ABS) mandibular bone defect model.
tensile testing, yielding a Young’s modulus of 310 MPa and consideration the silicone thickness before expansion)
a Poisson’s ratio of 0.34. Boundary conditions were set with after 6 weeks of immersion. This finding was used as the
both soft tissue and silicone membrane bottom-fixed. The mold parameter settings for subsequent expansion tests.
silicone expansion displacement was set as 0.5 mm, which Figure 7b illustrates the dimension changes during tablet
was referenced as the size increase for the swellable tablet expansion within the silicone membrane from weeks 1
device in the length/width/height directions as reported to 6. The tablets gradually swelled over time causing the
from the swelling test performed in section 2.1. Fabrication silicone membrane to enlarge to the intended volume size.
of swellable tablets and volume expansion test. In Figure 8, the relationship curve between the average
silicone expander slot expansion volume of five different
3. Results samples and the number of weeks indicated a fourth-
The swelling test results for the swellable tablets after 24 h degree polynomial curve; gradual growth in the first 2
of immersion reported expansion dimensions of 28 mm weeks, followed by continuous accelerated growth from
in length, 14 mm in width, and approximately 5 mm in the second to the fifth week, then returning to gradual
height. The overall expanded volume was 64 times the growth tendencies from the fifth to sixth week.
original volume. Figure 7a depicts swellable tablets at The expansion tests for the comma- and heart-shaped
compression, drying, and after immersion. Meanwhile, silicone expanders indicated that the average volumes of
the slot-shaped silicone expander achieved the anticipated five different samples after 6 weeks were 5815 and 6392
expansion volume of 1960 mm (28 × 14 × 5 mm; where mm , respectively. In comparison with the CAD models
3
3
5 mm was the average height after expansion, taking into for the comma- and heart-shaped expanders (i.e., with the
Volume 10 Issue 4 (2024) 572 doi: 10.36922/ijb.2918