Page 106 - ITPS-7-2
P. 106
INNOSC Theranostics and
Pharmacological Sciences Molecular docking against SARS-CoV-2
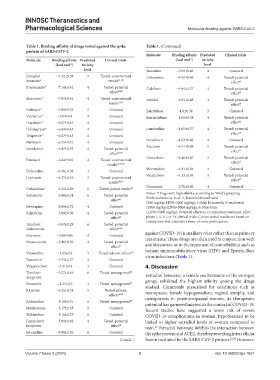
Table 1. Binding affinity of drugs tested against the spike Table 1. (Continued)
protein of SARS‑COV‑2
Molecule Binding affinity Predicted Clinical trials
Molecule Binding affinity Predicted Clinical trials (kcal mol ) toxicity
‑1
(kcal mol ) toxicity level
‑1
level Stavudine −5.04±0.43 4 Untested
Estradiol −7.22±0.58 5 Tested controversial Didanosine −4.92±0.45 4 Tested potential
,
,
benzoate* results - effect 49
23 26
Itraconazole* −7.14±0.42 4 Tested potential Cidofovir −4.84±0.27 4 Tested potential
,
,
effect 25,28 effect 47
Maraviroc* −7.07±0.41 4 Tested controversial Arbidol −4.81±0.48 4 Tested potential
,
,
results 31,32 effect 38
Indinavir* −7.04±0.32 5 Untested Zalcitabine −4.8±0.38 5 Untested
Vicriviroc* −7.03±0.4 4 Untested Emtricitabine −4.65±0.38 4 Tested potential
,
Dasabuvir* −6.87±0.47 4 Untested effect 42
Dolutegravir* −6.84±0.45 4 Untested Lamivudine −4.65±0.37 4 Tested potential
,
effect 50
Telaprevir* −6.67±0.43 4 Untested
Penciclovir −4.62±0.42 4 Untested
Nelfinavir −6.55±0.31 4 Untested
Aciclovir −4.51±0.43 5 Tested potential
,
,
Remdesivir −6.47±0.33 4 Tested potential effect 51
effect 32,38
,
Ritonavir −6.44±034 4 Tested controversial Ganciclovir −4.46±0.47 5 Tested potential
,
52
results 32,38,39 effect
Moroxydine −4.41±0.36 4 Untested
Delavirdine −6.38±0.38 4 Untested
Valaciclovir −4.33±0.44 5 Tested potential
,
Lopinavir −6.37±0.49 5 Tested controversial effect 47
,
results 38,39
Docosanol −3.76±0.42 4 Untested
Ceftazidime −6.22±0.29 6 Tested partial results 40
,
Notes: *Drugs with high affinity, according to Ward’s grouping.
,
Sofosbuvir −6.08±0.34 6 Tested potential Predicted toxicity level: 4: Harmful if swallowed
effect 41
(300 mg/kg<LD50<2000 mg/kg); 5: May be harmful if swallowed
Nevirapine −8.89±0.32 4 Untested (2000 mg/kg<LD50<5000 mg/kg); 6: Non-toxic
Rilpivirine −5.89±0.56 4 Tested potential (LD50>5000 mg/kg). Potential effects in co-infection treatment, pilot,
,
effect 42 phase I, II, III, or IV clinical trials. Controversial results are based on
conclusions that contradict those of some publications.
Tenofovir −5.67±0.29 6 Tested potential
,
alafenamide effect 43,44
Efavirenz −5.64±036 4 Untested against COVID-19 in auxiliary roles rather than as primary
treatments. These drugs are often used in conjunction with
Nitazoxanide −5.46±0.36 4 Tested potential
,
effect 45 antihistamines or in the treatment of comorbidities such as
,
Viramidine −5.35±0.3 5 Tested adverse effects 38 human immunodeficiency virus (HIV) and Epstein-Barr
virus infections (Table 1).
Zanamivir −5.33±0.37 5 Untested
Valganciclovir −5.31±0.4 5 Untested 4. Discussion
Tenofovir −5.27±0.49 6 Tested unsupported 44 Estradiol benzoate, a female sex hormone of the estrogen
,
disoproxil
Peramivir −5.27±0.3 4 Tested unsupported 46 group, exhibited the highest affinity among the drugs
,
studied. Commonly prescribed for conditions such as
,
Ribavirin −5.25±0.34 5 Tested adverse menopause, female hypogonadism, vaginal atrophy, and
effects 38,46
Zidovudine −5.19±0.31 6 Tested unsupported 47 osteoporosis in postmenopausal women, its therapeutic
,
potential has garnered interest in the context of COVID-19.
Methisazone −5.17±0.35 5 Untested Recent studies have suggested a lower risk of severe
Telbivudine −5.11±0.37 6 Untested COVID-19 complications in women, hypothesized to be
Famciclovir −5.09±0.49 4 Tested potential linked to higher estradiol levels in women compared to
,
favipiravir effect 48 men. Estradiol benzoate inhibits the interaction between
22
Edoxudine −5.08±0.36 6 Untested the spike protein and ACE2, thereby preventing intercellular
(Cont’d...) fusion mediated by the SARS-CoV-2 protein. 23,24 However,
Volume 7 Issue 2 (2024) 3 doi: 10.36922/itps.1651