Page 57 - ITPS-7-3
P. 57
INNOSC Theranostics and
Pharmacological Sciences Designing miRNA ONTs for cardiometabolic pandemics
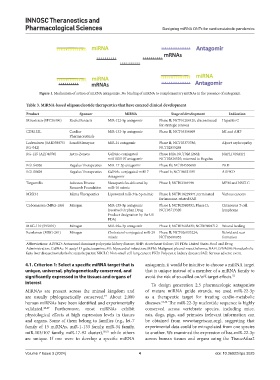
Figure 1. Mechanism of action of miRNA antagomirs. No binding of miRNA to complementary mRNAs in the presence of antagomir.
Table 3. MiRNA‑based oligonucleotide therapeutics that have entered clinical development
Product Sponsor MiRNA Stage of development Indication
Miravirsen (SPC36490) Roche/Santaris MiR-122-5p antagomir Phase II; NCT01200420; discontinued Hepatitis C
for strategic reasons
CDR132L Cardior MiR-132-3p antagomir Phase II; NCT05350969 MI and AHF
Pharmaceuticals
Lademirsen (SAR339375/ Sanofi/Genzyme MiR-21 antagomir Phase II; NCT03373786; Alport nephropathy
RG-012) NCT02855268
RG-125 (AZD4076) Astra-Zeneca GalNAc-conjugated Phase I/IIa; NCT02612662; NAFLD/NASH
miR103/107 antagomir NCT02826525; returned to Regulus
RGLS4326 Regulus Therapeutics MiR-17-5p antagomir Phase Ib; NCT04536688 PKD
RGLS8429 Regulus Therapeutics GalNAc-conjugated miR17 Phase1b; NCT06621191 ADPKD
Antagomir
TargomiRs Asbestos Disease Nanoparticles delivered by Phase I; NCT02369198 MPM and NSCLC
Research Foundation miR-16 mimic
MRX34 Mirna Therapeutics Liposomal miR-34a-5p mimic Phase I; NCT018229971; terminated Various cancers
for immune-related SAE
Cobomarsen (MRG-106) Miragen MiR-155-5p antagomir Phase I; NCT02580552; Phase II; Cutaneous T-cell
(received Orphan Drug NCT03713320 lymphoma
Product designation by the US
FDA)
MRG-110 (S95010) Miragen MiR-92a-3p antagomir Phase I; NCT03603431; NCT03494712 Wound healing
Remlarsen (MRG-201) Miragen Cholesterol-conjugated miR-29 Phase II; NCT026033224; Keloid and scar
mimic NCT03601052 formation
Abbreviations: ADPKD: Autosomal dominant polycystic kidney disease; AHF: Acute heart failure; US FDA: United States Food and Drug
Administration; GalNAc: N-acetyl-D-galactosamine; MI: Myocardial infarction; MPM: Malignant pleural mesothelioma; NAFLD/NASH: Nonalcoholic
fatty liver disease/nonalcoholic steatohepatitis; NSCLC: Non-small cell lung cancer; PKD: Polycystic kidney disease; SAE: Serious adverse event.
4.1. Criterion 1: Select a specific miRNA target that is antagomir, it would be intuitive to choose a miRNA target
unique, universal, phylogenetically conserved, and that is unique instead of a member of a miRNA family to
significantly expressed in the tissues and organs of avoid the risk of so-called on/off-target effects. 52
interest To design generation 2.5 pharmacologic antagonists
MiRNAs are present across the animal kingdom and of mature miRNA guide strands, we used miR-22-3p
are usually phylogenetically conserved. About 2,000 as a therapeutic target for treating cardio-metabolic
47
human miRNAs have been identified and experimentally diseases. 53,54 The miR-22-3p nucleotide sequence is highly
validated. 48,49 Furthermore, most miRNAs exhibit conserved across vertebrate species, including mice,
physiological effects at high expression levels in tissues rats, dogs, pigs, and primates (relevant information can
and organs. Some of them belong to families (e.g., let-7 be obtained from www.targetscan.org), suggesting that
family of 15 miRNAs, miR-1-133 family, miR-34 family, experimental data could be extrapolated from one species
miR-103/107 family, miR-17-92 cluster), 50,51 while others to another. We examined the expression of hsa-miR-22-3p
are unique. If one were to develop a specific miRNA across human tissues and organs using the TissueAtlas2
Volume 7 Issue 3 (2024) 4 doi: 10.36922/itps.3025