Page 90 - ITPS-8-1
P. 90
INNOSC Theranostics and
Pharmacological Sciences Brain glutamate level after treatment with NAC
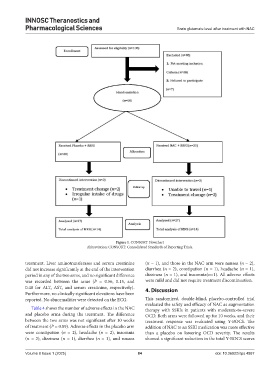
Figure 1. CONSORT Flowchart
Abbreviation: CONSORT: Consolidated Standards of Reporting Trials.
treatment. Liver aminotransferases and serum creatinine (n = 1), and those in the NAC arm were nausea (n = 2),
did not increase significantly at the end of the intervention diarrhea (n = 2), constipation (n = 1), headache (n = 1),
period in any of the two arms, and no significant difference dizziness (n = 1), and insomnia(n=1). All adverse effects
was recorded between the arms (P = 0.56, 0.15, and were mild and did not require treatment discontinuation.
0.48 for ALT, AST, and serum creatinine, respectively). 4. Discussion
Furthermore, no clinically significant elevations have been
reported. No abnormalities were detected on the ECG. This randomized, double-blind, placebo-controlled trial
evaluated the safety and efficacy of NAC as augmentation
Table 4 shows the number of adverse effects in the NAC therapy with SSRIs in patients with moderate-to-severe
and placebo arms during the treatment. The difference OCD. Both arms were followed up for 10 weeks, and their
between the two arms was not significant after 10 weeks treatment response was evaluated using Y-BOCS. The
of treatment (P = 0.89). Adverse effects in the placebo arm addition of NAC to an SSRI medication was more effective
were constipation (n = 2), headache (n = 2), insomnia than a placebo on lowering OCD severity. The results
(n = 2), dizziness (n = 1), diarrhea (n = 1), and nausea showed a significant reduction in the total Y-BOCS scores
Volume 8 Issue 1 (2025) 84 doi: 10.36922/itps.4887