Page 9 - ITPS-8-3
P. 9
INNOSC Theranostics and
Pharmacological Sciences AI in medical device safety
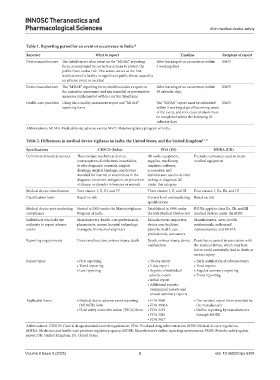
Table 1. Reporting period for an event or occurrence in India 14
Reporter What to report Timeline Recipient of report
Device manufacturer The initial report of an event on the “MDAE” reporting After learning of an occurrence; within MvPI
form, accompanied by corrective actions to protect the 5 working days
public from undue risk. This action serves as the first
notification of a fatality or significant public threat caused by
an adverse event or incident
Device manufacturer The “MDAE” reporting form, which includes a report on After learning of an occurrence; within MvPI
the causation assessment and any remedial or preventative 30 calendar days
measures implemented within a certain timeframe
Health-care providers Using the causality assessment report and “MDAE” The “MDAE” report must be submitted MvPI
reporting form within 5 working days of becoming aware
of the event, and root cause analysis must
be completed within the following 30
calendar days
Abbreviations: MDAE: Medical device adverse events; MvPI: Materiovigilance program of India.
Table 2. Differences in medical device vigilance in India, the United States, and the United Kingdom 14‑16
Specifications CDSCO (India) FDA (US) MHRA (UK)
Definition of medical devices These include mechanical devices, All tools, equipment, Excludes substances used to clean
contraceptives, disinfectants, insecticides, supplies, machinery, medical equipment
in vitro diagnostic materials, surgical implants, software,
dressings, surgical bandages, and devices accessories, and
intended for internal or external use in the disinfectants used in in vitro
diagnosis, treatment, mitigation, or prevention testing or diagnosis fall
of disease or disorder in humans or animals under this category
Medical device classification Four classes: I, II, III, and IV Three classes: I, II, and III Four classes: I, IIa, IIb, and III
Classification basis Based on risk Control level and marketing Based on risk
specifications
Medical device post-marketing Started in 2015 under the Materiovigilance Established in 1990 under PSURs apply to class IIa, IIb, and III
surveillance Program of India the Safe Medical Device Act medical devices under the MDR
Individuals who hold the Manufacturers, health-care professionals, Manufacturers, importers, Manufacturers, users, health
authority to report adverse pharmacists, nurses, hospital technology device user facilities, professionals, authorized
events managers, biomedical engineers patients, health-care representatives, and MHRA
professionals, consumers
Reporting requirements Device malfunction, serious injury, death Death, serious injury, device Event has occurred in association with
malfunction the medical device, which may have
led or could potentially lead to death or
serious injury
Report types • First reporting • 30‑day report • Early notification of adverse events
• Trend reporting • 5‑day report • Final reports
• Last reporting • Reports of individual • Regular summary reporting
adverse events • Trend reporting
• Initial report
• Additional reports:
Semiannual reports and
annual summary reports
Applicable forms • Medical device adverse event reporting • FDA 3500 • The incident report form provided by
(MDAER) form • FDA 3500A the manufacturer
• Field safety corrective action (FSCA) form • FDA 3419 • Online reporting by manufacturers
• FDA 3381 through MORE
• FDA 3417
Abbreviations: CDSCO: Central drugs standard control organization; FDA: Food and drug administration; MDR: Medical devices regulation;
MHRA: Medicines and health-care products regulatory agency; MORE: Manufacturer’s online reporting environment; PSUR: Periodic safety update
report; UK: United Kingdom; US: United States.
Volume 8 Issue 3 (2025) 3 doi: 10.36922/itps.6204