Page 74 - MI-2-3
P. 74
Microbes & Immunity Natural phage patentability in the U.S.
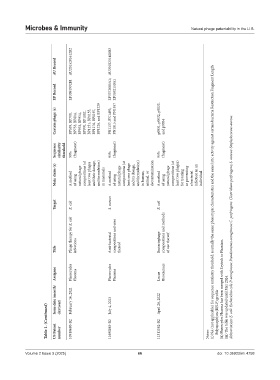
AU Record AU2015205512B2 AU2018231408B2
EP Record EP3091992B1 EP3372085A1; EP3592150A1
Certain phage (s) BP539, BP700, BP753, BP814, BP953, BP954, BP970, BP1002, BP1151, BP1155, BP1176, BP1197, BP1226, and BP1229 PN1137, PN1493, PN1815 and PN1957 p0031, p0032, p0033, and p0034
Sequence similarity threshold 99% (fragment) 95% (fragment) 95% (fragment)
Main claim (s) A method of using natural phage compositions (at least two phages and their dosage, and formulation) in mammals A method of using natural phage compositions (at least one phage and its dosage, and formulation) in human, animal, or decontamination A method of using natural phage compositions (at least two phages) for treating or preventing a bacterial infection in an individual
Target E. coli S. aureus E. coli (i) NA (not applicable) for sequence similarity threshold: normally the same phenotypic characteristics and the same lytic activity against certain bacteria Restriction Fragment Length
Phage therapy for E. coli compositions and uses Bacteriophage compositions and methods Abbreviation: E. coli: Escherichia coli; P. aeruginosa: Pseudomonas aeruginosa; C. perfringens: Clostridium perfringens; S. aureus: Staphylococcus aureus.
Title infections Anti-bacterial thereof of use thereof
Assignee Pherecydes Pharma Pherecydes Pharma Locus Biosciences
Issue date (month/ day/year) February 16, 2021 July 4, 2023 April 26, 2022 (ii) Pherecydes Pharma has been merged with Erytech to Phaxiam. (iii) The table was updated until May 2024.
Table 1. (Continued) US Patent number 10918680-B2 11690885-B2 11311582-B2 Notes: Polymorphism (RFLP) profile
Volume 2 Issue 3 (2025) 66 doi: 10.36922/mi.4758