Page 78 - MI-2-3
P. 78
Microbes & Immunity Natural phage patentability in the U.S.
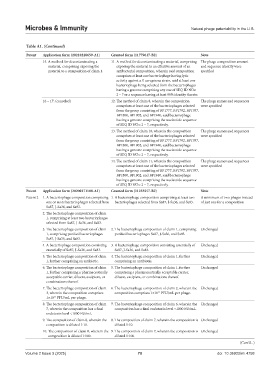
Table A1. (Continued)
Patent Application form (20210228659-A1) Granted form (11779617-B2) Note
15. A method for decontaminating a 11. A method for decontaminating a material, comprising The phage composition amount
material, comprising exposing the exposing the material to an effective amount of an and sequence identity were
material to a composition of claim 1. antibacterial composition, wherein said composition specified
comprises at least one bacteriophage having lytic
activity against a P. aeruginosa strain, said at least one
bacteriophage being selected from the bacteriophages
having a genome comprising any one of SEQ ID NOs:
2 – 7 or a sequence having at least 99% identity thereto.
16 – 17. (canceled) 12. The method of claim 8, wherein the composition The phage names and sequences
comprises at least one of the bacteriophages selected were specified
from the group consisting of BP1777, BP1792, BP1797,
BP1800, BP1902, and BP1940, said bacteriophage
having a genome comprising the nucleotide sequence
of SEQ ID NOs: 2 – 7, respectively.
13. The method of claim 10, wherein the composition The phage names and sequences
comprises at least one of the bacteriophages selected were specified
from the group consisting of BP1777, BP1792, BP1797,
BP1800, BP1902, and BP1940, said bacteriophage
having a genome comprising the nucleotide sequence
of SEQ ID NOs: 2 – 7, respectively.
14. The method of claim 11, wherein the composition The phage names and sequences
comprises at least one of the bacteriophages selected were specified
from the group consisting of BP1777, BP1792, BP1797,
BP1800, BP1902, and BP1940, said bacteriophage
having a genome comprising the nucleotide sequence
of SEQ ID NOs: 2 – 7, respectively.
Patent Application form (20200171108-A1) Granted form (11253557-B2) Note
Patent 2 1. A bacteriophage composition comprising 1. A bacteriophage composition comprising at least two A minimum of two phages instead
one or more bacteriophages selected from bacteriophages selected from Sa87, J-Sa36, and Sa83. of just one for a composition
Sa87, J-Sa36, and Sa83.
2. The bacteriophage composition of claim
1, comprising at least two bacteriophages
selected from Sa87, J-Sa36, and Sa83.
3. The bacteriophage composition of claim 2. The bacteriophage composition of claim 1, comprising Unchanged
1, comprising purified bacteriophages purified bacteriophages Sa87, J-Sa36, and Sa83.
Sa87, J-Sa36, and Sa83.
4. A bacteriophage composition consisting 3. A bacteriophage composition consisting essentially of Unchanged
essentially of Sa87, J-Sa36, and Sa83. Sa87, J-Sa36, and Sa83.
5. The bacteriophage composition of claim 4. The bacteriophage composition of claim 1, further Unchanged
1, further comprising an antibiotic. comprising an antibiotic.
6. The bacteriophage composition of claim 5. The bacteriophage composition of claim 1, further Unchanged
1, further comprising a pharmaceutically comprising a pharmaceutically acceptable carrier,
acceptable carrier, diluent, excipient, or diluent, excipient, or combinations thereof.
combinations thereof.
7. The bacteriophage composition of claim 6. The bacteriophage composition of claim 2, wherein the Unchanged
3, wherein the composition comprises composition comprises 1×10 PFU/mL per phage.
11
1×10 PFU/mL per phage.
11
8. The bacteriophage composition of claim 7. The bacteriophage composition of claim 6, wherein the Unchanged
7, wherein the composition has a final composition has a final endotoxin level <1000 EU/mL.
endotoxin level <1000 EU/mL.
9. The composition of claim 8, wherein the 8. The composition of claim 7, wherein the composition is Unchanged
composition is diluted 1:10. diluted 1:10.
10. The composition of claim 8, wherein the 9. The composition of claim 7, wherein the composition is Unchanged
composition is diluted 1:100. diluted 1:100.
(Cont’d...)
Volume 2 Issue 3 (2025) 70 doi: 10.36922/mi.4758