Page 169 - OR-1-3
P. 169
often fail to predict clinical outcomes due to oversimplified organoids faithfully preserve patient-specific genetic
cellular environments or interspecies discrepancies, leading mutations, ensuring that in vitro therapeutic responses
to high attrition rates in clinical trials. 4 closely mirror those observed in vivo. Furthermore, they
provide a complex, multicellular microenvironment,
Means et al. introduced an innovative approach that
5
integrates PDOs with high-throughput ASO screening, which is superior to traditional 2D cultures that often fail
to recapitulate the tissue-level effects of gene-targeting
establishing a methodology that significantly accelerates therapies. An additional advantage of this approach is its
the identification and validation of patient-specific ASO efficiency and scalability. The study’s methodology reduces
candidates. Published in Nature under the title Rapid the ASO validation timeline to merely 6 weeks, a significant
and Scalable Personalized ASO Screening in PDOs, this acceleration compared to conventional drug development
study showcases a streamlined workflow whereby cardiac pipelines. Such a rapid and adaptable workflow facilitates
organoids derived from Duchenne muscular dystrophy the customization of gene therapies at the individual
(DMD) patient-specific induced pluripotent stem cells level, expediting their transition into clinical applications.
(iPSCs) effectively recapitulate the cardiac dysfunction Beyond ASO therapeutics, organoids hold immense
characteristic of the disease. The study evaluates ASO- promise for screening an array of personalized treatments,
mediated dystrophin restoration, demonstrating that PDO- including small molecules, RNA-based drugs, and
based ASO screening constitutes a scalable and efficient clustered regularly interspaced short palindromic repeats
platform for precision medicine. This study establishes a rapid (CRISPR)-Cas9 gene-editing therapies. Given their high
and scalable platform for generating patient-derived cellular fidelity in replicating disease phenotypes, organoids are
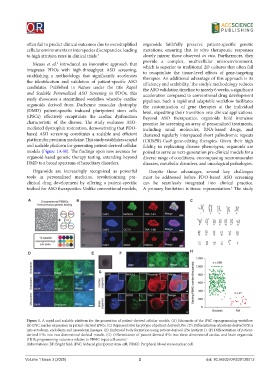
models (Figure 1A-H). The findings open new avenues for poised to serve as next-generation pre-clinical models for a
organoid-based genetic therapy testing, extending beyond diverse range of conditions, encompassing neuromuscular
DMD to a broad spectrum of hereditary disorders. diseases, metabolic disorders, and oncological pathologies.
Organoids are increasingly recognized as powerful Despite these advantages, several key challenges
tools in personalized medicine, revolutionizing pre- must be addressed before PDO-based ASO screening
clinical drug development by offering a patient-specific can be seamlessly integrated into clinical practice.
testbed for ASO therapeutics. Unlike conventional models, A primary limitation is tissue representation. The study
6
A B C
D E F H
G
Figure 1. A rapid and scalable platform for the generation of patient-derived cellular models. (A) Schematic of the iPSC reprogramming workflow.
(B) iPSC marker expression in patient-derived iPSCs. (C) Representative karyotype of patient-derived iPSs. (D) Differentiation of patient-derived iPSCs
into ectoderm, endoderm and mesoderm lineages. (E) Embryoid body formation using patient-derived iPSs (patient 1). (F) Differentiation of patient-
derived iPSs into two-dimensional skeletal muscle. (G) Differentiation of patient-derived iPSs into three-dimensional cardiac and brain organoids.
(H) Reprogramming outcomes relative to PBMC input cell counts. 5
Abbreviations: BF: Bright field; iPSC: Induced pluripotent stem cell; PBMC: Peripheral blood mononuclear cell.
Volume 1 Issue 3 (2025) 2 doi: 10.36922/OR025120012