Page 43 - {PDF Title}
P. 43
Weathering on Kamchatka volcanoes
out at atmospheric pressure by use of inert gas (most coefficient (LC). For low values of the mass ratio of
often, argon). The ICP-AES method is widely utilized in rock to solution, that is, for x m << 1, Equation I takes
i∙
geology because it allows simultaneous determination the simpler form:
of a large number of macro- and micro-components. C ≈ k ∙x ∙m. (II)
Meanwhile, elements could be in a dissolved sample i i i
within wide detection limits (from tenths of ppm to tens The initial masses of the samples were the same,
of thousands of ppm). allowing us to calculate the values of the relative LC
At a constant initial content of organic acid in the of microelements k as the ratio C /C* , where C* is
i
i
i
i
solution, the concentration dependence C , of the the microelement concentration after distilled water
i
leached microelement i (μg/L) on the mass ratio of rock extraction without ultrasound shaking.
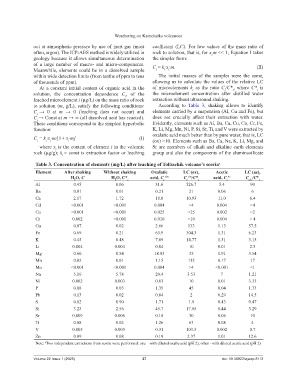
to solution (m, g/L), satisfy the following conditions: According to Table 3, shaking allows to identify
C → 0 at m → 0 (leaching does not occur) and elements carried by a suspension (Al, Cu and Fe), but
i
C → Const at m → ∞ (all dissolved acid has reacted). does not crucially affect their extraction with water.
i
These conditions correspond to the simplest hyperbolic Evidently, elements such as Al, Ba, Ca, Cu, Co, Cr, Fe,
function: K, Li, Mg, Mn, Ni, P, Si, Sr, Ti, and V were extracted by
C = k ∙x ∙m/[1+ x ∙m] (I) oxalatic acid much better than by pure water, that is, LC
(ox) >10. Elements such as Ba, Ca, Na, K, Li, Mg, and
i
i
i
i
where x is the content of element i in the volcanic Sr are members of alkali and alkaline earth elements
i
rock (μg/g); k = const is extraction factor or leaching group and also the components of the aluminosilicate
i
Table 3. Concentration of elements (mg/L) after leaching of Tolbachik volcano’s scoria a
Element After shaking Without shaking Oxalatic LC (ox), Acetic LC (ac),
H O, C H O, С* i acid, C i Ox C /C* i acid, C i Ac C /C* i
Ox
2
2
iAc
i
Al 0.45 0.06 31.6 526.7 5.4 90
Ba 0.01 0.01 0.21 21 0.06 6
Ca 2.17 1.72 18.8 10.93 11.0 6.4
Cd <0.001 <0.001 0.004 >4 0.004 >4
Co <0.001 <0.001 0.025 >25 0.002 >2
Cr 0.002 <0.001 0.010 >10 0.004 > 4
Cu 0.07 0.02 2.66 133 1.15 57.5
Fe 0.69 0.21 63.9 304.3 1.31 6.23
K 0.45 0.48 7.09 14.77 1.51 3.15
Li 0.004 0.004 0.04 10 0.01 2.5
Mg 0.60 0.54 18.93 35 1.91 3.54
Mn 0.02 0.01 1.15 115 0.17 17
Mo <0.001 <0.001 0.004 >4 <0.001 <1
Na 5.16 5.78 20.4 3.53 7 1.21
Ni 0.002 0.003 0.03 10 0.01 3.33
P 0.08 0.03 1.35 45 0.04 1.33
Pb 0.13 0.02 0.04 2 0.29 14.5
S 0.82 0.90 1.71 1.9 0.43 0.47
Si 3.23 2.56 45.7 17.85 8.44 3.29
Sr 0.009 0.006 0.18 30 0.06 10
Ti 0.08 0.02 1.26 63 0.08 4
V 0.005 0.003 0.31 103.3 0.002 0.7
Zn 0.09 0.08 0.19 2.37 1.01 12.6
Note: Two independent extractions from scoria were performed: one – with diluted oxalic acid (pH 2); other - with diluted acetic acid (pH 2).
a
Volume 22 Issue 1 (2025) 37 doi: 10.36922/ajwep.8113