Page 57 - AN-1-3
P. 57
Advanced Neurology Targets and medications for AVM of CNS
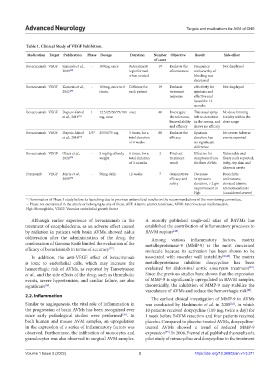
Table 1. Clinical Study of VEGF Inhibition.
Medication Target Publication Phase Dosage Duration Number Objective Result Side effect
of cases
Bevacizumab VEGF Simonds et al., - 100mg, once Retreatment 19 Evaluate the Frequency Not displayed
2009 [30] is performed effectiveness and severity of
when needed bleeding was
decreased
Bevacizumab VEGF Karnezis et al., - 100mg, once to 8 Different for 19 Evaluate effectively for Not displayed
2012 [29] times, each patient treatment epistaxis and
response effective and
lasted for 12
months
Bevacizumab VEGF Dupuis-Girod 1 12.5/25/50/75/100 once 40 Investigate This nasal spray No dose limiting
et al., 2014 [31] mg, once the tolerance, left no detection toxicity within the
bioavailability in the serum, and dose range
and efficacy shows no efficacy
Bevacizumab VEGF Dupuis-Girod 2/3* 25/50/75 mg 3 times, for a 80 Evaluate the Epistaxis No severe Adverse
et al., 2016 [32] total duration efficacy duration has events reported
of 4 weeks no significant
difference
Bevacizumab VEGF Olsen et al., - 5 mg/kg of body 6 times, for a 2 Find out Effective for Vulnerable and
2020 [33] weight total duration treatment symptoms from flossy nails reported;
of 3 months result the liver AVMs itchy, dry skin and
alopecia areata
Pazopanib VEGF Marie et al., - 50mg daily 12 weeks 7 demonstrate Decrease Bronchitis
2019 [35] efficacy and in epistaxis and nausea;
safety duration, > 2 gm elevated alanine
improvement in aminotransferase
Hgb (considered severe)
*: Termination of Phase 3 study before its launching due to previous unbeneficial results on the recommendations of the monitoring committee,
–: Phase not mentioned in the article or belonging to any of them, ALT: Alanine aminotransferase, AVM: Arteriovenous malformation,
Hgb: Hemoglobin, VEGF: Vascular endothelial growth factor
Although earlier experience of bevacizumab in the A recently published single-cell atlas of BAVMs has
treatment of encephaledema, as an adverse effect caused established the contribution of inflammatory processes to
by radiation in patients with brain AVMs, showed nidus BAVM rupture .
[40]
obliteration after the administration of the drug, the Among various inflammatory factors, matrix
combination of Gamma Knife limited the evaluation of the metalloproteinase-9 (MMP-9) is the most concerned
efficacy of bevacizumab in terms of accuracy . molecule because its activation has been shown to be
[37]
In addition, the anti-VEGF effect of bevacizumab associated with vascular wall instability [41,42] . The matrix
is toxic to endothelial cells, which may increase the metalloproteinase inhibitor doxycycline has been
[43]
hemorrhagic risk of AVMs, as reported by Tanvetyanon evaluated for abdominal aortic aneurysm treatment .
et al., and the side effects of the drug, such as thrombotic Since the previous studies have shown that the expression
events, severe hypertension, and cardiac failure, are also of MMP-9 is significantly upregulated in BAVM samples,
significant . theoretically, the inhibition of MMP-9 may stabilize the
[38]
vasculature of AVMs and reduce the hemorrhagic risk .
[44]
2.2. Inflammation
The earliest clinical investigation of MMP-9 in AVMs
Similar to angiogenesis, the vital role of inflammation in was conducted by Hashimoto et al. in 2005 , in which
[22]
the progression of brain AVMs has been recognized ever 10 patients received doxycycline (100 mg, twice a day) for
since early pathological studies were performed . In 1 week before BAVM resection and four patients received
[39]
both human and mouse AVM samples, an upregulation placebo. Compared to placebo-treated AVMs, doxycycline-
in the expression of a series of inflammatory factors was treated AVMs showed a trend of reduced MMP-9
observed. Furthermore, the infiltration of monocytes and expression . In 2008, Frenzel et al. published the results of a
[45]
granulocytes was also observed in surgical AVM samples. pilot study of minocycline and doxycycline in the treatment
Volume 1 Issue 3 (2022) 3 https://doi.org/10.36922/an.v1i3.211