Page 81 - AN-1-3
P. 81
Advanced Neurology Microglia in autism spectrum disorder
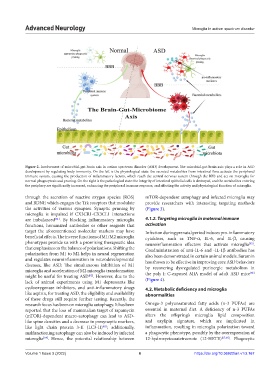
Figure 2. Involvement of microbial-gut-brain axis in autism spectrum disorder (ASD) development. The microbial-gut-brain axis plays a role in ASD
development by regulating body immunity. On the left is the physiological state: the secreted metabolites from intestinal flora activate the peripheral
immune system, causing the production of inflammatory factors, which reach the central nervous system through the BBB and act on microglia for
normal phagocytosis and pruning. On the right is the pathological state: the integrity of intestinal epithelial cells is destroyed, and the metabolites entering
the periphery are significantly increased, enhancing the peripheral immune response, and affecting the activity and physiological function of microglia.
through the secretion of reactive oxygen species (ROS) mTOR-dependent autophagy and infected microglia may
and BDNF, which engages the Trk receptors that modulate provide researchers with interesting targeting methods
the activities of various synapses. Synaptic pruning by (Figure 3).
microglia is impaired if CX3CR1-CX3CL1 interactions
are imbalanced . By blocking inflammatory microglia 4.1.2. Targeting microglia in maternal immune
[61]
functions, humanized antibodies or other reagents that activation
target the aforementioned molecular markers may have Infection during prenatal period induces pro-inflammatory
beneficial effects. The inverse functions of M1/M2 microglia cytokines, such as TNF-α, IL-6, and IL-β, causing
phenotypes provide us with a promising therapeutic idea neuroinflammation effectors that activate microglia .
[65]
that emphasizes on the balance of polarizations. Shifting the Coadministration of anti-IL-6 and -IL-1β antibodies has
polarization from M1 to M2 helps in neural regeneration also been demonstrated in certain animal models. Suramin
and regulates neuroinflammation in neurodevelopmental has shown to be effective in improving core ASD behaviors
diseases, like ASD. The simultaneous inhibition of M1 by recovering dysregulated purinergic metabolism in
microglia and acceleration of M2 microglia transformation [66]
might be useful for treating ASD . However, due to the the poly I: C-exposed MIA model of adult ASD mice
[62]
(Figure 4).
lack of animal experiments using M1 depressants like
cyclooxygenase inhibitors, and anti-inflammatory drugs 4.2. Metabolic deficiency and microglia
like aspirin, for treating ASD, the eligibility and availability abnormalities
of these drugs still require further testing. Recently, the
research focus has been on microglia autophagy. It has been Omega-3 polyunsaturated fatty acids (n-3 PUFAs) are
reported that the loss of mammalian target of rapamycin essential in maternal diet. A deficiency of n-3 PUFAs
(mTOR)-dependent macro-autophagy can lead to ASD- alters the offspring’s microglia lipid composition
like spine densities and a decrease in presynaptic markers, and oxylipin signature, which are implicated in
like light chain protein 3-II (LC3-II) ; additionally, inflammation, resulting in microglia polarization toward
[63]
malfunctioning autophagy can also be induced by infected a phagocytic phenotype, possibly by the overexpression of
microglia . Hence, the potential relationship between 12-hydroxyeicosatetraenoic (12-HETE) [67,68] . Phagocytic
[64]
Volume 1 Issue 3 (2022) 6 https://doi.org/10.36922/an.v1i3.167