Page 29 - AN-3-1
P. 29
Advanced Neurology BTKi in brain diseases
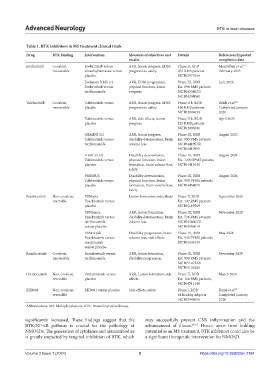
Table 1. BTK inhibitors in MS treatment clinical trials
Drug BTK binding Intervention Measures of objectives and Details References/Expected
results completion date
Evobrutinib Covalent, Evobrutinib versus ARR, lesion progress, EDSS Phase II, RDB Montalban et al. 17
irreversible dimethylfumarate versus progression, safety 267 RMS patients February 2025
placebo NCT02975349
Evolution RMS 1/2 ARR, EDSS progression, Phase III, RDB June 2026
Evobrutinib versus physical function, lesion Est. 898 RMS patients
teriflunomide progress NCT04338022/
NCT04338061
Tolebrutinib Covalent, Tolebrutinib versus ARR, lesion progress, EDSS Phase II b, RDB Reich et al. 42
irreversible placebo progression, safety 130 RMS patients Completed January
NCT03889639 2020
Tolebrutinib versus ARR, side effects, lesion Phase II b, RDB April 2025
placebo progress 125 RMS patients
NCT03996291
GEMINI 1/2 ARR, lesion progress, Phase III, RDB August 2023
Tolebrutinib versus disability deterioration, brain Est. 900 RMS patients
teriflunomide volume loss NCT04410978/
NCT04410991
HERCULES Disability deterioration, Phase III, RDB August 2024
Tolebrutinib versus physical function, lesion Est. 1290 SPMS patients
placebo formation, brain volume loss, NCT04411641
safety
PERSEUS Disability deterioration, Phase III, RDB August 2024
Tolebrutinib versus physical function, lesion Est. 990 PPMS patients
placebo formation, brain volume loss, NCT04458051
safety
Fenebrutinib Non-covalent, FENopta Lesion formation, side effects Phase II, RDB September 2026
reversible Fenebrutinib versus Est. 102 RMS patients
placebo NCT05119569
FENhance ARR, lesion formation, Phase III, RDB November 2025
Fenebrutinib versus disability deterioration, brain Est. 736 RMS patients
teriflunomide volume loss NCT04586023/
versus placebo NCT04586010
FENtrepid Disability progression, brain Phase III, RDB May 2028
Fenebrutinib versus volume loss, side effects Est. 946 PPMS patients
ocrelizumab NCT04544449
versus placebo
Remibrutinib Covalent, Remibrutinib versus ARR, lesion formation, Phase III, RDB November 2029
irreversible teriflunomide disability progression, Est. 800 RMS patients
NCT05147220/
NCT05156281
Orelabrutinib Non-covalent, Orelabrutinib versus ARR, Lesion formation, side Phase II, RDB March 2024
reversible placebo effects Est. 160 RMS patients,
NCT04711148
BIIB091 Non-covalent, BIIB091 versus placebo Side effects, safety Phase I, RDB Bame et al. 57
reversible 64 healthy subjects Completed January
NCT03943056 2020
Abbreviations: MS: Multiple sclerosis; BTK: Bruton’s tyrosine kinase.
significantly increased. These findings suggest that the may successfully prevent CNS inflammation and the
BTK/NF-κB pathway is crucial for the pathology of advancement of illness. 65-67 Hence, apart from holding
NMOSDs. The generation of cytokines and autoantibodies potential as an MS treatment, BTK inhibitors could also be
is greatly impacted by targeted inhibition of BTK, which a significant therapeutic intervention for NMOSD.
Volume 3 Issue 1 (2024) 5 https://doi.org/10.36922/an.2184