Page 73 - AN-4-2
P. 73
Advanced Neurology Brain bioavailability of targeted protein degraders
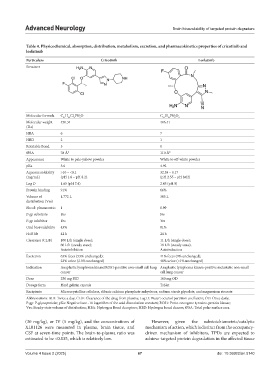
Table 4. Physicochemical, absorption, distribution, metabolism, excretion, and pharmacokinetics properties of crizotinib and
lorlatinib
Particulars Crizotinib Lorlatinib
Structure
Molecular formula C H Cl FN O C H FN O
21 22 2 5 21 19 6 2
Molecular weight 450.34 406.41
(Da)
HBA 6 7
HBD 2 1
Rotatable Bond 5 0
tPSA 78 Ų 110 Ų
Appearance White to pale-yellow powder White to off-white powder
pKa 5.6 4.92
Aqueous solubility >10 – <0.1 32.38 – 0.17
(mg/mL) (pH 1.6 – pH 8.2). (pH 2.55 – pH 8.02)
Log D 1.65 (pH 7.4) 2.45 (pH 9)
Protein binding 91% 66%
Volume of 1,772 L 305 L
distribution (Vss)
Blood: plasma ratio 1 0.99
P-gp substrate Yes No
P-gp inhibitor Yes Yes
Oral bioavailability 43% 81%
Half life 42 h 24 h
Clearance (CL/F) 100 L/h (single dose); 11 L/h (single dose);
60 L/h (steady state); 18 L/h (steady state);
Autoinhibition Autoinduction
Excretion 63% feces (53% unchanged); 41% feces (9% unchanged);
22% urine (2.3% unchanged) 48% urine (<1% unchanged)
Indication Anaplastic lymphoma kinase/ROS1-positive non-small cell lung Anaplastic lymphoma kinase-positive metastatic non-small
cancer cell lung cancer
Dose 250 mg BID 100 mg OD
Dosage form Hard gelatin capsule Tablet
Excipients Microcrystalline cellulose, dibasic calcium phosphate anhydrous, sodium starch glycolate, and magnesium stearate
Abbreviations: BID: Twice a day; CL/F: Clearance of the drug from plasma; Log D: Water: octanol partition coefficient; OD: Once daily;
P-gp: P-glycoprotein; pKa: Negative base -10 logarithm of the acid dissociation constant; ROS1: Proto-oncogene tyrosine-protein kinase;
Vss: Steady-state volume of distribution; HBA: Hydrogen Bond Acceptors; HBD: Hydrogen bond donors; tPSA: Total polar surface area.
(30 mg/kg), or IV (5 mg/kg), and the concentrations of However, given the substoichiometric/catalytic
XL01126 were measured in plasma, brain tissue, and mechanism of action, which is distinct from the occupancy-
CSF at seven-time points. The brain-to-plasma ratio was driven mechanism of inhibitors, TPDs are expected to
estimated to be <0.035, which is relatively low. achieve targeted protein degradation in the affected tissue
Volume 4 Issue 2 (2025) 67 doi: 10.36922/an.5140