Page 31 - EJMO-9-3
P. 31
Eurasian Journal of
Medicine and Oncology Role of common CXC chemokines in NAFLD
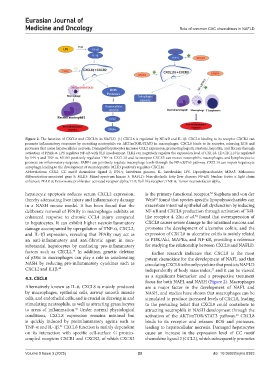
Figure 2. The function of CXCL8 and CXCL10 in NAFLD. (1) CXCL8 is regulated by NF-κB and IL-1β. CXCL8 binding to its receptor CXCR2 can
promote inflammatory responses by recruiting neutrophils via AKT/mTOR/STAT3 in macrophages. CXCL8 binds to its receptor, releasing ROS and
proteases that cause hepatocellular necrosis. Damaged hepatocytes increase CCL2 expression, promoting hepatic steatosis, hepatitis, and fibrosis through
activation of PPAR-α. LPS regulates NF-κB with TLR involvement. TLR2 can negatively regulate the expression level of CXCL8. (2) CXCL10 is regulated
by IFN-γ and TNF-α. MDA5 positively regulates TNF-α. CXCL10 and its receptor CXCR3 can recruit neutrophils, macrophages, and lymphocytes to
promote an inflammatory response. FABP4 can positively regulate macrophage levels through the NF-κB/P65 pathway. CXCL10 can impair hepatocyte
autophagy, leading to the development of steatohepatitis. MLK3 positively regulates CXCL10.
Abbreviations: CCL2: CC motif chemokine ligand 2; IFN-γ: Interferon gamma; IL: Interleukin; LPS: Lipopolysaccharide; MDA5: Melanoma
differentiation-associated gene 5; MLK3: Mixed-spectrum kinase 3; NAFLD: Non-alcoholic fatty liver disease; NF-κB: Nuclear factor κ light chain
enhancer; PPAR-α: Peroxisome proliferator-activated receptor alpha; TLR: Toll-like receptor; TNF-α: Tumor necrosis factor alpha.
52
hepatocyte apoptosis reduces serum CXCL2 expression, is the primary functional receptor. Stephens and von der
thereby attenuating liver injury and inflammatory damage Weid found that species-specific lipopolysaccharides can
53
in a NASH mouse model. It has been found that the exacerbate intestinal epithelial cell dysfunction by inducing
deliberate removal of PPARγ in macrophages exhibits an NF-κB and CXCL8 production through activation of Toll-
54
enhanced response to chronic CCL4 injury compared like receptor 4. Zhu et al. found that overexpression of
to hepatocytes. It can exhibit higher necroinflammatory CXCL8 causes severe damage to the intestinal mucosa and
damage accompanied by upregulation of TNF-α, CXCL2, promotes the development of ulcerative colitis, and the
and IL-1β expression, revealing that PPARγ may act as expression of CXCL8 in ulcerative colitis is mainly related
an anti-inflammatory and anti-fibrotic agent in non- to PI3K/Akt, MAPKs, and NF-κB, providing a reference
substantial hepatocytes by mediating pro-inflammatory for studying the relationship between CXCL8 and NAFLD.
factors such as CXCL2. In addition, genetic deletion Earlier research indicates that CXCL8 is the most
48
of p38α in macrophages can play a role in ameliorating potent chemokine for the development of NAFL and that
NASH by reducing pro-inflammatory cytokines such as circulating CXCL8 is the only cytokine that predicts NAFLD
CXCL2 and IL1β. 49 independently of body mass index, and it can be viewed
55
4.3. CXCL8 as a significant biomarker and a prospective treatment
focus for both NAFL and NASH (Figure 2). Macrophages
Alternatively known as IL-8, CXCL8 is mainly produced are a major factor in the development of NAFL and
by macrophages, epithelial cells, airway smooth muscle NASH, and studies have shown that macrophages can be
cells, and endothelial cells, and is crucial in drawing in and stimulated to produce increased levels of CXCL8, leading
stimulating neutrophils, as well as attracting granulocytes to the prevailing belief that CXCL8 could contribute to
to areas of inflammation. Under normal physiological attracting neutrophils in NASH development through the
50
conditions, CXCL8 expression remains minimal but activation of the AKT/mTOR/STAT3 pathway. CXCL8
56
is quickly induced by proinflammatory agents such as binds to its receptor and releases ROS and proteases,
TNF-α and IL-1β. CXCL8 function is mainly dependent leading to hepatocellular necrosis. Damaged hepatocytes
51
on its interaction with specific cell-surface G protein- cause an increase in the expression level of CC motif
coupled receptors CXCR1 and CXCR2, of which CXCR2 chemokine ligand 2 (CCL2), which subsequently promotes
Volume 9 Issue 3 (2025) 23 doi: 10.36922/ejmo.8383