Page 15 - GPD-3-1
P. 15
Gene & Protein in Disease Enhancing fertility with CRISPR
3.4. Producing genetically modified animals for In a recent study aiming to create human models of liver
research cancer, researchers utilized the CRISPR/Cas9 system to
In the rapidly advancing medical and pharmaceutical induce loss-of-function mutations in PTEN and P53 genes
landscape, a substantial gap exists between the potential in monkeys. CRISPR demonstrated significant promise
of gene editing technologies and their practical application in generating tissue models of human liver cancer, aiding
86
in clinical trials. However, CRISPR/Cas9 facilitates the in drug discovery and development. Similarly, monkeys
generation of model organisms, bridging the divide between with biallelic mutations of P53 were produced using
proof-of-concept studies in small animals such as rodents CRISPR/Cas9, closely mimicking genetic malfunctions in
and human clinical trials. In biomedical research, a variety humans and potentially offering avenues for curing human
81
87
of animals, including zebrafish, mice, rats, monkeys, frogs, genetic disorders. In addition, animal models generated
rabbits, dogs, pigs, and cats, serve as experimental models. 82-85 through CRISPR/Cas9, such as Dnajb7 knockout mice,
Non-human primate (NHP) models, due to their strong revealed no defects in reproductive health, indicating that
resemblance to humans, are particularly advantageous and Dnajb7 is not necessary for mouse fertility. 88
can be generated by genetically altering NHP zygotes. 82
3.5. Potential for gene therapy in reproductive
Researchers have developed protocols for assisted disorders
reproduction and genetic alteration in NHPs, incorporating
techniques such as ICSI, ovarian stimulation, in vitro CRISPR technology holds significant promise for enhancing
oocyte maturation, IVF, embryo culture, and embryo reproductive health in various ways. It can be employed
transfer. Genetic engineering methods, including gene to rectify harmful genetic and reproductive disorders,
knockout and knockin techniques using gene editing ultimately contributing to improved reproductive health
protocols, have been employed alongside emerging gene and the prevention of certain genetic and reproductive
editing methods for creating genetically modified NHP issues (Figure 3). CRISPR gene therapy has demonstrated
49
models for biomedical research. 71 efficacy in treating diverse diseases and genetic problems,
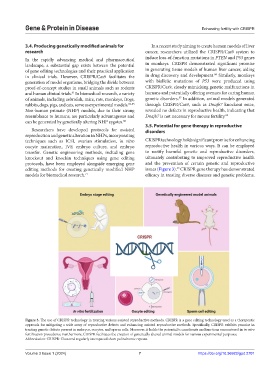
Figure 3. The use of CRISPR technology in treating various assisted reproductive methods. CRISPR is a gene editing technology used as a therapeutic
approach for mitigating a wide array of reproductive defects and enhancing assisted reproductive methods. Specifically, CRISPR exhibits promise in
treating genetic defects present in embryos, oocytes, and sperm cells. Moreover, it holds the potential to ameliorate malfunctions encountered in in vitro
fertilization procedures. Furthermore, CRISPR facilitates the creation of genetically altered animal models for various experimental purposes.
Abbreviation: CRISPR: Clustered regularly interspaced short palindromic repeats.
Volume 3 Issue 1 (2024) 7 https://doi.org/10.36922/gpd.2701