Page 47 - GTM-2-1
P. 47
Global Translational Medicine Inflammatory and anti-inflammatory responses in HTLV1
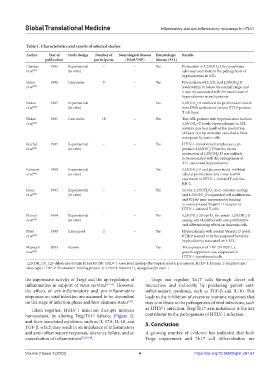
Table 1. Characteristics and results of selected studies
Author Year of Study design Number of Neurological disease Hematologic Results
publication participants (HAM/TSP) disease (ATL)
Champs 1986 Experimental – – Yes Production of 1,25(OH )D by lymphoma
2
et al. [47] (in vitro) cells may contribute to the pathogenesis of
hypercalcemia in ATL.
Satou 1986 Case series 5 – Yes Five patients with ATL had 1,25(OH )D
2
et al. [53] levels within or below the normal range, and
it was not associated with the usual cause of
hypercalcemia in such patients.
Nakao 1987 Experimental – – Yes 1,25(OH ) D inhibited the proliferation and de
2
et al. [62] (in vitro) novo DNA synthesis of certain HTLV-positive
T cell lines.
Nakao 1987 Case series 18 – Yes Two ATL patients with hypercalcemia had low
et al. [62] 1,25(OH ) D levels. Hypercalcemia in ATL
2
patients may be a result of the production
of factor (s) that stimulate osteoclastic bone
resorption by tumor cells.
Reichel 1987 Experimental – – Yes HTLV-1-transformed lymphocytes can
et al. [63] (in vitro) produce 1,25(OH ) D but the excess
2
production of 1,25(OH ) D was unlikely
2
to be associated with the pathogenesis of
ATL-associated hypercalcemia.
Koizumi 1989 Experimental – – Yes 1,25(OH ) D and glucocorticoid-inhibited
2
et al. [64] (in vitro) cellular proliferation and c-myc mRNA
expression in HTLV-1-infected T-cell line,
KH-2.
Inoue 1993 Experimental – – Yes 22-oxa-1,25(OH )D (non-calcemic analog)
3
2
et al. [65] (in vitro) and 1,25(OH) D suppressed cell proliferation
2
and PTHrP gene expression by binding
to overexpressed Vitamin D receptor in
HTLV-1-infected T cells.
Elstner 1994 Experimental - – Yes 1,25(OH )-20-epi-D the potent 1,25(OH ) D
2
3,
2
et al. [66] (in vitro) analog, was identified with anti-proliferative
and differentiating effects on leukemic cells.
Peter 1995 Case report 2 – Yes Hypercalcemia with normal Vitamin D levels.
et al. [67] PTHrP seemed to be the supposed factor for
hypercalcemia associated with ATL.
Masutani 2005 Review – – Yes The expression of TBP-2/VDUP1, a
et al. [68] growth suppressor, was suppressed in
HTLV-1-transformed cells.
1,25(OH ) D: 1,25-dihydroxyvitamin D; HAM/TSP: HTLV-1-associated myelopathy/tropical spastic paraparesis; HTLV-1: Human T-lymphotropic
2
virus type 1; TBP-2: Thioredoxin-binding protein-2; VDUP1: Vitamin D upregulated protein 1
3
the suppressive activity of Tregs and the up regulation of Tregs can regulate Th17 cells through direct cell
inflammation in support of virus survival [52-55] . However, interaction and indirectly by producing potent anti-
the effects of anti-inflammatory and pro-inflammatory inflammatory cytokines, such as TGF-β and IL-10. This
responses on viral infection are assumed to be dependent leads to the inhibition of excessive immune responses that
on the stage of infection phase and host immune status [4,7] . may contribute to the pathogenesis of viral infections, such
Taken together, HTLV-1 infection disrupts immune as HTLV-1 infection. Treg/Th17 axis imbalance is the key
homeostasis, by altering Treg/Th17 balance (Figure 2), contributor to the pathogenesis of HTLV-1 infection.
and their associated cytokines, such as IL-17A, IL-10, and 5. Conclusion
TGF-β, which may result in an imbalance of inflammatory
and anti-inflammatory responses, tolerance failure, and an A growing number of evidence has indicated that both
exacerbation of inflammation [13,56-60] . Tregs impairment and Th17 cell differentiation are
Volume 2 Issue 1 (2023) 4 https://doi.org/10.36922/gtm.v2i1.67