Page 89 - GTM-2-3
P. 89
Global Translational Medicine HPLC-UV lacosamide quantification
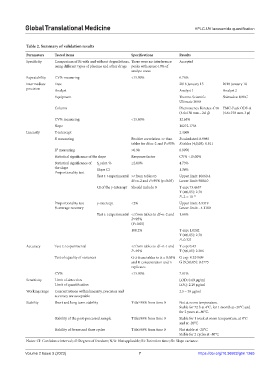
Table 2. Summary of validation results
Parameters Tested items Specifications Results
Specificity Comparison of Rt with and without degradations, There were no interference Accepted
using different types of plasmas and other drugs peaks with areas>10% of
analyte areas
Repeatability CV% measuring <15.00% 6.76%
Intermediate Date 2018 January 15 2018 January 18
precision Analyst Analyst 1 Analyst 2
Equipment Thermo Scientific Shimadzu UFLC
Ultimate 3000
Column Phenomenex Kinetex-C18 YMC-Pack ODS-A
(4.6×150 mm – 2.6 μ) (4.6×150 mm-3 μ)
CV% measuring <15.00% 12.64%
Slope 10272.1754
Linearity Y-intercept 2.4069
R measuring Positive correlation >r than R calculated: 0.9985
tables for df=n-2 and P=95% R tables (4;0,05): 0.811
R measuring >0.98 0.9970
2
Statistical significance of the slope Response factor CV% <15.00%
Statistical significance of S relat. % ≤2.00% 4.73%
b
the slope Slope CI 1.36%
Proportionality test
Test t: t experimental >r from tables to Upper limit: 10660.4
df=n-2 and P=95% (p<0.05) Lower limit: 9884.0
CI of the y-intercept Should include 0 T exp: 73.4657
T (4;0,05): 2.78
P=2 × 10 -16
Proportionality test y-intercept <2% Upper limit: 8.9319
% average recovery Lower limit: - 4.1180
Test t: t experimental <t from tables to df=n-2 and 1.66%
P=95%
(P>0.05)
100.2% T exp: 1.0242
T (4;0,05): 2.78
P=0.321
Accuracy Test t: t experimental <t from tables to df=n-1 and T exp: 0.43
P=95% T (8;0,05): 2.306
Test of equality of variances G<t from tables to α = 0.05% G exp: 0.324609
and K concentration and n G (9;3;0,05): 0.4775
replicates
CV% <15.00% 7.01%
Sensitivity Limit of detection LOD: 0.69 µg/ml
Limit of quantification LOQ: 2.29 µg/ml
Working range Concentrations within linearity, precision and 2.5 – 30 µg/ml
accuracy are acceptable
Stability Short and long-term stability Title≥90% from time 0 Not at room temperature.
Stable for 72 h at 4°C, for 1 month at−20°C and
for 2 years at−80°C.
Stability of the post-processed sample Title≥90% from time 0 Stable for 1 week at room temperature, at 4°C
and at -20°C
Stability of freeze and thaw cycles Title≥90% from time 0 Not stable at -20°C
Stable for 2 cycles at -80°C
Notes: CI: Confidence interval; df: Degrees of freedom; N/A: Not applicable; Rt: Retention time; Sb: Slope variance.
Volume 2 Issue 3 (2023) 7 https://doi.org/10.36922/gtm.1265