Page 115 - GTM-4-2
P. 115
Global Translational Medicine Platelet aggregation inhibition by 2-isoxazolines
The activation of platelets by adding ADP leads to a change our results with other compounds containing a similar
in the conformation of membrane receptors, and GPIIa/IIIb heterocycle showed a relatively equal ability to inhibit
6
is responsible for further aggregation. Samples of PRPR with platelet aggregation. The degree of inhibition of the
added effectors were kept at room temperature, followed by compounds we obtained was 21 – 43% at 5 mmol/L and
an addition of a solution of labeled antibodies CD41a-FITC 29 – 56% at 10 mmol/L. The inhibitory ability of the
(antibodies to the GPIIa receptor) and CD61-PE (antibodies previous compounds was in the range of 35 – 65% at a
to the GPIIIb receptor). Subsequently, an analysis was concentration of 4.4 – 4.5 mmol/L.
performed, and the number of double-positive cells (CD41a While the simplicity of the obtained compounds may
+
CD61 ) was counted and compared with the number of the not suggest the presence of exceptional properties in
+
same cells in the control sample, which did not contain any
inhibitors. The obtained data on the ability to inhibit platelet platelet aggregation, we believe the data hold significant
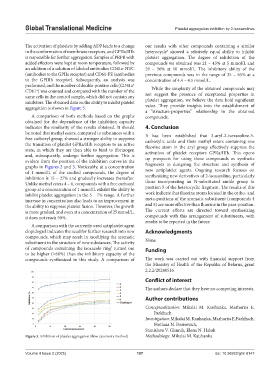
aggregation is shown in Figure 3. value. They provide insights into the establishment of
a “structure-properties” relationship in the obtained
A comparison of both methods based on the graphs compounds.
obtained for the dependence of the inhibition capacity
indicates the similarity of the results obtained. It should 4. Conclusion
be noted that methyl esters, compared to substances with a It has been established that 3-aryl-2-isoxazoline-5-
free carboxyl group, showed a stronger ability to suppress carboxylic acids and their methyl esters containing one
the transition of platelet GPIIa/IIIb receptors to an active fluorine atom in the aryl group effectively suppress the
state, in which they are then able to bind to fibrinogen activation of platelet receptors GPIIa/IIIb. This opens
and, subsequently, undergo further aggregation. This is up prospects for using these compounds as synthetic
evident from the position of the inhibition curves in the fragments in designing the structure and synthesis of
graphs in Figures 2 and 3. Noticeably, at a concentration
of 1 mmol/L of the studied compounds, the degree of new antiplatelet agents. Ongoing research focuses on
inhibition is 15 – 27% and gradually increases thereafter. synthesizing new derivatives of 2-isoxazoline, particularly
Unlike methyl esters 4 – 6, compounds with a free carboxyl those incorporating an N-substituted amide group in
group at a concentration of 1 mmol/L exhibit the ability to position 5 of the heterocyclic fragment. The results of this
inhibit platelet aggregation in the 5 – 7% range. A further work indicate that fluorine atoms located in the ortho- and
increase in concentration also leads to an improvement in meta-positions of the aromatic substituent (compounds 4
the ability to suppress platelet fusion. However, the growth and 5) are more effective than fluorine in the para-position.
is more gradual, and even at a concentration of 25 mmol/L, The current efforts are directed toward synthesizing
it does not reach 50%. compounds with this arrangement of substituents, with
results to be reported in the future.
A comparison with the currently used antiplatelet agent
clopidogrel indicates the need for further research into new Acknowledgments
compounds, which may result in modifying the aromatic
substituent in the structure of new substances. The activity None.
of compounds containing the isoxazole ring turned out Funding
7
to be higher (≥60%) than the inhibitory capacity of the
compounds synthesized in this study. A comparison of The work was carried out with financial support from
the Ministry of Health of the Republic of Belarus, grant
2.2.2/20240516.
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
Conceptualization: Mikalai M. Kauhanka, Marharita E.
Parkhach
Investigation: Mikalai M. Kauhanka, Marharita E.Parkhach,
Svetlana N. Borisevich,
Stanislava V. Glinnik, Elena N. Haluk
Figure 3. Inhibition of platelet aggregation (flow cytometry method) Methodology: Mikalai M. Kauhanka
Volume 4 Issue 2 (2025) 107 doi: 10.36922/gtm.8147