Page 193 - IJB-10-1
P. 193
International Journal of Bioprinting Nanoclay biopolymer inks for 3D printing
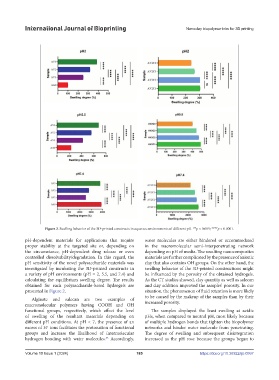
Figure 2. Swelling behavior of the 3D-printed constructs in aqueous environments of different pH. **p < 0.005; ****p < 0.0001.
pH-dependent materials for applications that require water molecules are either hindered or accommodated
proper stability at the targeted site or, depending on in the macromolecular semi-interpenetrating network
the circumstance, pH-dependent drug release or even depending on pH of media. The resulting nanocomposites
controlled dissolvability/degradation. In this regard, the materials are further complicated by the presence of anionic
pH sensitivity of the novel polysaccharide materials was clay that also contains OH groups. On the other hand, the
investigated by incubating the 3D-printed constructs in swelling behavior of the 3D-printed constructions might
a variety of pH environments (pH = 2, 5.5, and 7.4) and be influenced by the porosity of the obtained hydrogels.
calculating the equilibrium swelling degree. The results As the CT studies showed, clay quantity as well as salecan
obtained for each polysaccharide-based hydrogels are and clay addition improved the samples’ porosity. In our
presented in Figure 2. situation, the phenomenon of fluid retention is more likely
Alginate and salecan are two examples of to be caused by the makeup of the samples than by their
macromolecular polymers having COOH and OH increased porosity.
functional groups, respectively, which affect the level The samples displayed the least swelling at acidic
of swelling of the resultant materials depending on pHs, when compared to neutral pH, most likely because
different pH conditions. At pH < 7, the presence of an of multiple hydrogen bonds that tighten the biopolymer
excess of H ions facilitates the protonation of functional networks and hinder water molecule from penetrating.
+
groups and increase the likelihood of intermolecular The degree of swelling and subsequent disintegration
hydrogen bonding with water molecules. Accordingly, increased as the pH rose because the groups began to
55
Volume 10 Issue 1 (2024) 185 https://doi.org/10.36922/ijb.0967