Page 135 - IJB-10-4
P. 135
International Journal of Bioprinting 3D printing innovations against infection
to the potential threat of the implant, but also represents a relatively low risk of infection. However, if bacterial
the physiological response of the host organism attempting cells attach to the surface of the 3D-printed antimicrobial
to protect itself. 48,49 When biomaterials are embedded in a material before host cells do, the host is considered
mammalian host in the form of a foreign body, initiating losing in the race, and the bacteria will form a biofilm
an intricate “race for the surface,” a competition ensues on the surface, inevitably leading to implant-associated
between host cells and contaminating bacteria to occupy infections. Therefore, understanding the mechanism of
50
the implant surface. The rapid integration of biomaterials biofilm formation is crucial in the design and selection of
into host tissues, a key factor for the success of many 3D implant materials. Biofilm formation is a dynamic and
implants, is also supported by evidence indicating that complex process involving various cell types, molecular
prompt integration plays a crucial role in preventing signals, biological responses, and factors such as material
bacterial adhesion and colonization. Briefly, if the host surface and culture conditions. 51,52 This process can be
cells are able to take the lead in occupying the available broadly divided into three components: biofilm formation,
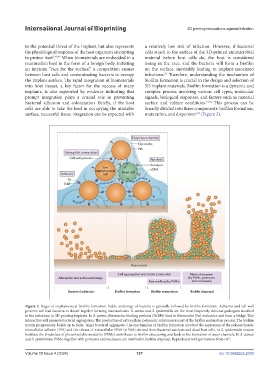
surface, successful tissue integration can be expected with maturation, and dispersion (Figure 2).
4,53
Figure 2. Stages of staphylococcal biofilm formation. Stable anchorage of bacteria is generally followed by biofilm formation. Adhesins and cell wall
proteins will lead bacteria to cluster together forming microcolonies. S. aureus and S. epidermidis are the most frequently detected pathogens involved
in the infections in 3D-printing implants. In S. aureus, fibronectin-binding proteins (FnBPs) bind to fibronectin (Fn) molecules and form a bridge. This
interaction will promote bacterial aggregation. The production of extracellular polymeric substances is part of the biofilm maturation process. The biofilm
matrix progressively builds up to form larger bacterial aggregates. The mechanisms of biofilm formation involved the expression of the polysaccharide
intercellular adhesin (PIA) and the release of extracellular DNA (eDNA) derived from bacterial autolysis and dead host cells. In S. epidermidis mature
biofilms, the β-subclass of phenol-soluble modulins (PSMs) contributes to biofilm structuring and leads to the formation of water channels. In S. aureus
and S. epidermidis, PSMs, together with proteases and nucleases, are involved in biofilm dispersal. Reproduced with permission from ref. . 4
Volume 10 Issue 4 (2024) 127 doi: 10.36922/ijb.2338