Page 285 - IJB-10-4
P. 285
International Journal of Bioprinting 3D printing prosthesis for palatal fistula
Elastomer in the compression test (p < 0.01). The tensile models and the finished printed products (Figure 2B).
2
moduli of both elastomers (Figure 7F) are not statistically Finally, the prosthesis was implanted into the animal
different (p > 0.05). Subsequently, in the compression model’s palate defect, and the prosthesis was appropriately
test, the compressive strength (Figure 7G) and Young’s positioned. The fistula was repaired accurately (Figure S1 in
modulus (Figure 7H) of Elastomer were higher than Supplementary File). The prosthesis is shown in Figure S2
4
those of Elastomer (p < 0.01). Thus, we consider that the (Supplementary File).
2
mechanical properties of Elastomer are more potent than
4
those of Elastomer . 3.7. Biocompatibility tests of PU elastomers
2
The oral environment is in constant motion, because 3.7.1. In vitro cytocompatibility and morphological
of actions such as speaking, chewing, swallowing, and evaluation of L929 cells
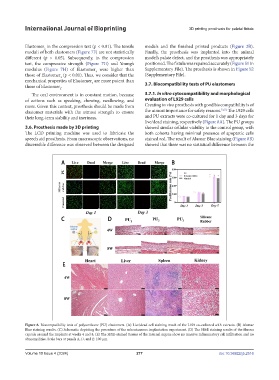
more. Given this context, prosthesis should be made from Creating in vivo prosthesis with good biocompatibility is of
elastomer materials with the utmost strength to ensure the utmost importance for safety reasons. 51,52 The L929 cells
their long-term stability and inertness. and PU extracts were co-cultured for 1 day and 3 days for
live/dead staining, respectively (Figure 8A). The PU groups
3.6. Prosthesis made by 3D printing showed similar cellular viability to the control group, with
The LCD printing machine was used to fabricate the both cohorts having minimal presence of apoptotic cells
speech aid prosthesis. From macroscopic observations, no stained red. The result of Alamar Blue staining (Figure 8B)
discernible difference was observed between the designed showed that there was no statistical difference between the
Figure 8. Biocompatibility tests of polyurethane (PU) elastomers. (A) Live/dead cell staining result of the L929 co-cultured with extracts. (B) Alamar
Blue staining results. (C) Schematic depicting the procedure of the subcutaneous implantation experiment. (D) The H&E staining results of the fibrous
capsule around the implants at weeks 4 and 8. (E) The H&E-stained tissues of the internal organs show no massive inflammatory cell infiltration and no
abnormalities. Scale bars at panels A, D, and E: 100 µm.
Volume 10 Issue 4 (2024) 277 doi: 10.36922/ijb.2516