Page 333 - IJB-10-4
P. 333
International Journal of Bioprinting 3D bioprinting of composite hydrogels
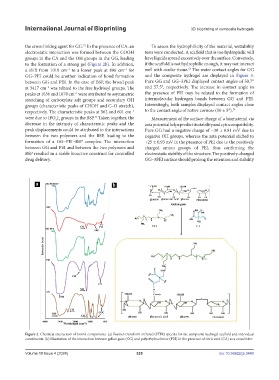
the cross-linking agent for GG. In the presence of CA, an To assess the hydrophilicity of the material, wettability
72
electrostatic interaction was formed between the COOH tests were conducted. A scaffold that is too hydrophilic will
groups in the CA and the OH groups in the GG, leading have liquids spread excessively over the surface. Conversely,
to the formation of a strong gel (Figure 2b). In addition, if the scaffold is not hydrophilic enough, it may not interact
68
a shift from 1010 cm to a lower peak at 896 cm for well with ocular tissue. The water contact angles for GG
−1
−1
GG–PEI could be another indication of bond formation and the composite hydrogel are displayed in Figure 3.
between GG and PEI. In the case of BSP, the broad peak Pure GG and GG–3PEI displayed contact angles of 50.7°
at 3417 cm was related to the free hydroxyl groups. The and 57.5°, respectively. The increase in contact angle in
−1
peaks at 1636 and 1078 cm were attributed to asymmetric the presence of PEI may be related to the formation of
−1
stretching of carboxylate salt groups and secondary OH intermolecular hydrogen bonds between GG and PEI.
groups (characteristic peaks of CHOH and C–O stretch), Interestingly, both samples displayed contact angles close
respectively. The characteristic peaks at 562 and 601 cm to the contact angle of native corneas (50 ± 5°). 74
−1
were due to (PO ) groups in the BSP. Taken together, the Measurement of the surface charge of a biomaterial via
73
4 3
decrease in the intensity of characteristic peaks and the zeta potential helps predict its stability and cytocompatibility.
peak displacements could be attributed to the interactions Pure GG had a negative charge of −30 ± 0.81 mV due to
between the two polymers and the BSP, leading to the negative OH groups, whereas the zeta potential shifted to
formation of a GG–PEI–BSP complex. The interaction +25 ± 0.93 mV in the presence of PEI due to the positively
between GG and PEI and between the two polymers and charged amino groups of PEI, thus confirming the
BSP resulted in a stable bioactive construct for controlled electrostatic stability of the structure. The positively-charged
drug delivery. GG–3PEI surface should prolong the retention and stability
Figure 2. Chemical interaction of bioink components. (a) Fourier-transform infrared (FTIR) spectra for the composite hydrogel scaffold and individual
constituents. (b) Illustration of the interaction between gellan gum (GG) and polyethyleneimine (PEI) in the presence of citric acid (CA) as a crosslinker.
Volume 10 Issue 4 (2024) 325 doi: 10.36922/ijb.3440