Page 337 - IJB-10-4
P. 337
International Journal of Bioprinting 3D bioprinting of composite hydrogels
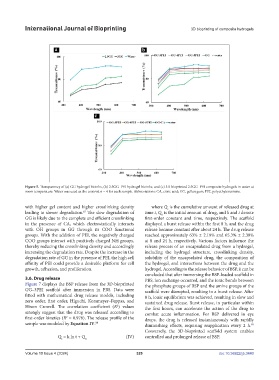
Figure 5. Transparency of (a) GG hydrogel bioinks, (b) 2.5GG–PEI hydrogel bioinks, and (c) 3D bioprinted 2.5GG–PEI composite hydrogels in water at
room temperature. Water was used as the control; n = 4 for each sample. Abbreviations: CA, citric acid; GG, gellan gum; PEI, polyethyleneimine.
with higher gel content and higher crosslinking density where Q is the cumulative amount of released drug at
t
leading to slower degradation. The slow degradation of time t, Q is the initial amount of drug, and k and t denote
87
0
GG is likely due to the complete and efficient crosslinking first-order constant and time, respectively. The scaffold
in the presence of CA, which electrostatically interacts displayed a burst release within the first 8 h, and the drug
with OH groups in GG through its COO functional release became constant after about 24 h. The drug release
groups. With the addition of PEI, the negatively charged reached approximately 63% ± 2.19% and 65.3% ± 2.39%
COO groups interact with positively charged NH groups, at 8 and 24 h, respectively. Various factors influence the
thereby reducing the crosslinking density and accordingly release process of an encapsulated drug from a hydrogel,
increasing the degradation rate. Despite the increase in the including the hydrogel structure, crosslinking density,
degradation rate of GG in the presence of PEI, the high cell solubility of the encapsulated drug, the composition of
affinity of PEI could provide a desirable platform for cell the hydrogel, and interactions between the drug and the
growth, adhesion, and proliferation. hydrogel. According to the release behavior of BSP, it can be
concluded that after immersing the BSP-loaded scaffold in
3.6. Drug release PBS, ion exchange occurred, and the ionic bonds between
Figure 7 displays the BSP release from the 3D-bioprinted the phosphate groups of BSP and the amine groups of the
GG–3PEI scaffold after immersion in PBS. Data were scaffold were disrupted, resulting in a burst release. After
fitted with mathematical drug release models, including 8 h, ionic equilibrium was achieved, resulting in slow and
zero order, first order, Higuchi, Korsmeyer–Peppas, and sustained drug release. Burst release, in particular within
Hixon–Crowell. The correlation coefficient (R ) values the first hours, can accelerate the action of the drug to
2
strongly suggest that the drug was released according to combat acute inflammation. For BSP delivered in eye
first-order kinetics (R = 0.976). The release profile of the drops, the drug is released instantaneously with rapidly
2
sample was modeled by Equation IV. diminishing effects, requiring reapplication every 2 h.
88
89
Conversely, the 3D-bioprinted scaffold system enables
Q = k ln t + Q 0 (IV) controlled and prolonged release of BSP.
t
Volume 10 Issue 4 (2024) 329 doi: 10.36922/ijb.3440