Page 408 - IJB-10-5
P. 408
International Journal of Bioprinting DEX-Loaded PLGA microspheres enhance cartilage regeneration
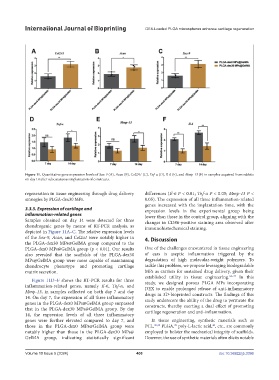
Figure 11. Quantitative gene expression levels of Sox-9 (A), Acan (B), Col2A1 (C), Tnf-α (D), Il-6 (E), and Mmp-13 (F) in samples acquired from rabbits
on day 14 after subcutaneous implantation of constructs.
regeneration in tissue engineering through drug delivery differences (Il-6 P < 0.01; Tnf-α P < 0.05; Mmp-13 P <
strategies by PLGA-dex30 MPs. 0.05). The expression of all three inflammation-related
genes increased with the implantation time, with the
3.3.5. Expression of cartilage and expression levels in the experimental group being
inflammation-related genes lower than those in the control group, aligning with the
Samples obtained on day 14 were detected for three changes in CD86-positive staining area observed after
chondrogenic genes by means of RT-PCR analysis, as immunohistochemical staining.
depicted in Figure 11A–C. The relative expression levels
of the Sox-9, Acan, and Col2a1 were notably higher in 4. Discussion
the PLGA-dex30 MPs@GelMA group compared to the
PLGA-dex0 MPs@GelMA group (p < 0.01). Our results One of the challenges encountered in tissue engineering
also revealed that the scaffolds of the PLGA-dex30 of ears is aseptic inflammation triggered by the
MPs@GelMA group were more capable of maintaining degradation of high molecular-weight polymers. To
chondrocyte phenotype and promoting cartilage tackle this problem, we propose leveraging biodegradable
matrix secretion. MPs as carriers for sustained drug delivery, given their
established utility in tissue engineering. 34–37 In this
Figure 11D–F shows the RT-PCR results for three study, we designed porous PLGA MPs incorporating
inflammation-related genes, namely Il-6, Tnf-α, and DEX to enable prolonged release of anti-inflammatory
Mmp-13, in samples collected on both day 7 and day drugs in 3D-bioprinted constructs. The findings of this
14. On day 7, the expression of all three inflammatory study underscore the ability of the drug to permeate the
genes in the PLGA-dex0 MPs@GelMA group surpassed constructs, thereby exerting a dual effect of promoting
that in the PLGA-dex30 MPs@GelMA group. By day cartilage regeneration and anti-inflammation.
14, the expression levels of all three inflammatory
genes were further elevated compared to day 7, and In tissue engineering, synthetic materials such as
39
40
those in the PLGA-dex0 MPs@GelMA group were PCL, 30,38 PLGA, poly-L-lactic acid, , etc., are commonly
notably higher than those in the PLGA-dex30 MPs@ employed to bolster the mechanical integrity of scaffolds.
GelMA group, indicating statistically significant However, the use of synthetic materials often elicits notable
Volume 10 Issue 5 (2024) 400 doi: 10.36922/ijb.3396