Page 16 - IJB-10-6
P. 16
International Journal of Bioprinting 3D-bioprinted multicellular lung organoids
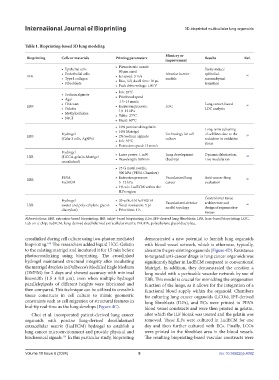
Table 1. Bioprinting-based 3D lung modeling
Mimicry or
Bioprinting Cells or materials Printing parameters Results Ref.
improvement
• Piezoelectric nozzle:
• Epithelial cells 80 µm sized Easily induce
• Endothelial cells Alveolar barrier epithelial-
IBB • Jet speed: 3 m/s 86
• Type I collagen • Rise, fall, dwell time: 10 µs models mesenchymal
• Fibroblasts transition
• Peak drive voltage: ±80 V
• Ink: 28°C
• Sodium alginate • Printhead speed
• Agar :13–15 mm/s
• Chitosan Lung cancer-based
EBB • Extrusion pressure: LOC 98
• Gelatin 10–12 kPa LOC analysis
• Methylcellulose • Table: 27°C
• NaCl
• Head: 60°C
• 10% porcine skin gelatin Long-term culturing
• 10% Matrigel
Hydrogel Technology for cell of cell lines due to the
EBB • 2% Sodium alginate 99
(Calu-3 cells, AgNPs) culture reduction in oxidative
• Ink: 35°C stress
• Extrusion speed: 13 mm/s
Hydrogel
LBB (HCCA-gelatin-Matrigel • Laser power: 1 mW Lung development Dynamic fabrication, 100
(bud tip)
• Wavelength: 800 nm
Live modulation
crosslinked)
• 25 G metal nozzle,
500 kPA (PEVA Chamber)
PEVA • Extrusion pressure: Vascularized lung Anti-cancer drug
EBB 101
LudECM 5–12 kPa cancer evaluation
• 1% w/v LudECM within the
ILFs region
Control over tissue
Hydrogel • 20 wt%, 6 kDa PEGDA
LBB (water and poly-ethylene glycol- • Voxel resolution: 5 pl Vascularized alveolar architecture and 105
design of regenerative
model topology
diacrylate) • Print time: 1 h
tissues
Abbreviations: EBB, extrusion-based bioprinting; IBB, inkjet-based bioprinting; ILFs, IPF-derived lung fibroblasts; LBB, laser-based bioprinting; LOC,
Lab-on-a-chip; LudECM, lung-derived decellularized extracellular matrix; PEGDA, polyethylene glycol diacrylate.
crosslinked during cell culture using two-photon mediated demonstrated a new potential to furnish lung organoids
100
bioprinting. The researchers added liquid HCC-Gelatin with blood vessel network, which is otherwise, typically,
to the existing matrigel and incubated it for 15 min before absent in the pre-existing organoids (Figure 4D). Resistance
photocrosslinking using bioprinting. The crosslinked to targeted anti-cancer drugs in lung cancer organoids was
hydrogel maintained structural integrity after incubating significantly higher in LudECM compared to conventional
the matrigel droplets in Dulbecco‘s Modified Eagle Medium Matrigel. In addition, they demonstrated the creation a
(DMEM) for 2 days and showed accuracy with minimal lung model with a perfusable vascular network by use of
linewidth (1.5 ± 0.8 µm), even when multiple hydrogel EBB. This model is crucial for mimicking the oxygenation
parallelepipeds of different heights were fabricated and function of the lungs, as it allows for the integration of a
then compared. This technique can be utilized to crosslink functional blood supply within the organoid. Chambers
tissue constructs in cell culture to mimic geometric for culturing lung cancer organoids (LCOs), IPF-derived
constraints such as cell migration or structural features in lung fibroblasts (ILFs), and ECs were printed in PEVA
bud-tip real-time as the lung develops (Figure 4C). blood vessel constructs and were then printed in gelatin,
Choi et al. incorporated patient-derived lung cancer after which the ILF bioink was treated and the gelatin was
organoids with porcine lung-derived decellularized removed. These ILFs were cultured in LudECM for one
extracellular matrix (LudECM) hydrogel to establish a day and then further cultured with ECs. Finally, LCOs
lung cancer microenvironment and provide physical and were printed in the fibroblast area in the blood vessels.
biochemical signals. In this particular study, bioprinting The resulting bioprinting-based vascular constructs were
101
Volume 10 Issue 6 (2024) 8 doi: 10.36922/ijb.4092