Page 133 - IJB-7-1
P. 133
Chow, et al.
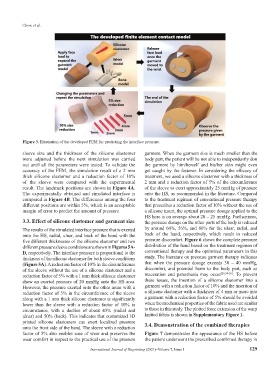
Figure 3. Illustration of the developed FEM for predicting the interface pressure.
sleeve size and the thickness of the silicone elastomer garment. When the garment size is much smaller than the
were adjusted before the next simulation was carried body part, the patient will be not able to independently don
out until all the parameters were tested. To validate the the garment by him/herself and his/her skin might even
accuracy of the FEM, the simulation result of a 2 mm get caught by the fastener. In considering the efficacy of
thick silicone elastomer and a reduction factor of 10% treatment, we used a silicone elastomer with a thickness of
of the sleeve were compared with the experimental 2 mm and a reduction factor of 5% of the circumference
result. The landmark positions are shown in Figure 4A. of the sleeve to exert approximately 25 mmHg of pressure
The experimentally obtained and simulated interface is onto the HS, as recommended in the literature. Compared
compared in Figure 4B. The differences among the four to the treatment regimen of conventional pressure therapy
different positions are within 5%, which is an acceptable that prescribes a reduction factor of 10% without the use of
margin of error to predict the amount of pressure. a silicone insert, the optimal pressure dosage applied to the
HS here is on average about 20 – 25 mmHg. Furthermore,
3.3. Effect of silicone elastomer and garment size the pressure dosage on the other parts of the body is reduced
The results of the simulated interface pressure that is exerted by around 60%, 56%, and 80% for the ulnar, radial, and
onto the HS, radial, ulnar, and back of the hand with the back of the hand, respectively, which result in reduced
five different thicknesses of the silicone elastomer and two pressure discomfort. Figure 6 shows the complete pressure
different pressure sleeve conditions are shown in Figures 5A- distribution of the hand based on the treatment regimen of
D, respectively. The interface pressure is proportional to the conventional therapy and the optimized parameters in this
thickness of the silicone elastomer for both sleeve conditions study. The literature on pressure garment therapy indicates
(Figure 5A). A reduction factor of 10% in the circumference that when the pressure dosage exceeds 30 – 40 mmHg,
of the sleeve without the use of a silicone elastomer and a discomfort, and potential harm to the body part, such as
reduction factor of 5% with a 1 mm thick silicone elastomer maceration and paresthesia may occur [26,54-56] . To prevent
show an exerted pressure of 20 mmHg onto the HS area. these issues, the insertion of a silicone elastomer into a
However, the pressure exerted onto the other areas with a garment with a reduction factor of 10% and the insertion of
reduction factor of 5% in the circumference of the sleeve a silicone elastomer with a thickness of 4 mm or more into
along with a 1 mm thick silicone elastomer is significantly a garment with a reduction factor of 5% should be avoided
lower than the sleeve with a reduction factor of 10% in when the mechanical properties of the fabric used are similar
circumstance, with a decline of about 43% (radial and to those in this study. The plotted force extension of the warp
ulnar) and 50% (back). This indicates that customized 3D knitted fabric is shown in Supplementary Figure 1.
printed silicone elastomers can exert localized pressure 3.4. Demonstration of the combined therapies
onto the front side of the hand. The sleeve with a reduction
factor of 5% also enables ease of wear and preserves the Figure 7 demonstrates the appearance of the HS before
wear comfort in respect to the practical use of the pressure the patient underwent the prescribed combined therapy in
International Journal of Bioprinting (2021)–Volume 7, Issue 1 129