Page 302 - IJB-8-4
P. 302
Vessel-on-a-Chip for Antiangiogenic Drug Screening
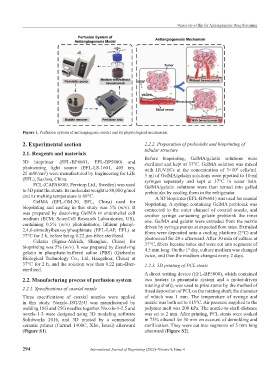
Figure 1. Perfusion system of antiangiogenic model and its physiological mechanism.
2. Experimental section 2.2.2. Preparation of prebioinks and bioprinting of
tubular structure
2.1. Reagents and materials
Before bioprinting, GelMA/gelatin solutions were
3D bioprinter (EFL-BP6601, EFL-BP5800) and sterilized and kept at 37°C. GelMA solution was mixed
photocuring light source (EFL-LS-1601, 405 nm, with HUVECs at the concentration of 3×10 cells/ml.
6
25 mW/cm ) were manufactured by Engineering for Life 3 ml of GelMA/gelatin solutions were pipetted to 10-ml
2
(EFL), Suzhou, China. syringes separately and kept at 37°C in water bath.
PCL (CAPA6800, Perstorp Ltd., Sweden) was used GelMA/gelatin solutions were then turned into gelled
to 3D print the stents. Its molecular weight is 80,000 g/mol prebioinks by cooling them in the refrigerator.
and its melting temperature is 60°C. A 3D bioprinter (EFL-BP6601) was used for coaxial
GelMA (EFL-GM-30, EFL, China) used for bioprinting. A syringe containing GelMA prebioink was
bioprinting and casting in this study was 5% (w/v). It connected to the outer channel of coaxial nozzle, and
was prepared by dissolving GelMA in endothelial cell another syringe containing gelatin prebioink the inner
medium (ECM; ScienCell Research Laboratories, US), one. GelMA and gelatin were extruded from the nozzle
containing 0.5% (w/v) photoinitiator, lithium phenyl- driven by syringe pumps at expected flow rates. Extruded
2,4,6-trimethylbenzoylphosphinate (EFL-LAP, EFL) at fibers were deposited onto a cooling platform (2°C) and
37°C for 2 h, before being 0.22 μm-filter-sterilized. photocured for 20 s afterward. After 30 min of culture at
Gelatin (Sigma-Aldrich, Shanghai, China) for
bioprinting was 5% (w/v). It was prepared by dissolving 37°C, fibers became tubes and were cut into segments of
4.5 mm long. On the 1 day, culture medium was changed
st
gelatin in phosphate-buffered saline (PBS) (Qizhenhu twice, and then the medium changed every 2 days.
Biological Technology Co., Ltd, Hangzhou, China) at
37°C for 2 h, and the solution was then 0.22 μm-filter- 2.2.3. 3D printing of PCL stents
sterilized.
A direct writing device (EFL-BP5800), which contained
2.2. Manufacturing process of perfusion system two heaters (a pneumatic system and a motor-driven
rotating shaft), was used to print stents by the method of
2.2.1. Specifications of coaxial nozzle fused deposition of PCL on the rotating shaft; the diameter
Three specifications of coaxial nozzles were applied of which was 1 mm. The temperature of syringe and
in this study. Nozzle-18G/25G was manufactured by nozzle was both set to 115°C. Air pressure supplied to the
welding 18G and 25G needles together. Nozzle-1-2.5 and polymer melt was 200 kPa. The nozzle-to-shaft-distance
nozzle-1-3 were designed using 3D modeling software was set to 2 mm. After printing, PCL stents were soaked
Solidworks 2016, and 3D printed by a commercial in 75% ethanol for 30 min on account of demolding and
ceramic printer (Carmel 1400C, XJet, Israel) afterward sterilization. They were cut into segments of 5 mm long
(Figure S1). afterward (Figure S2).
294 International Journal of Bioprinting (2022)–Volume 8, Issue 4