Page 288 - IJB-9-1
P. 288
International Journal of Bioprinting 3D-Printed scaffolds
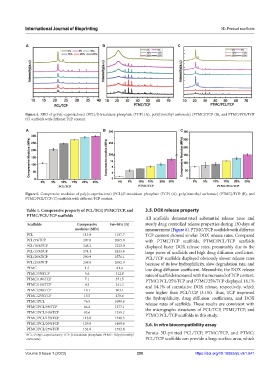
A B C
Figure 4. XRD of poly(ε-caprolactone) (PCL)/β-tricalcium phosphate (TCP) (A), poly(trimethyl carbonate) (PTMC)/TCP (B), and PTMC/PCL/TCP
(C) scaffolds with different TCP content.
A B C
Figure 5. Compressive modulus of poly(ε-caprolactone) (PCL)/β-tricalcium phosphate (TCP) (A), poly(trimethyl carbonate) (PTMC)/TCP (B), and
PTMC/PCL/TCP (C) scaffolds with different TCP content.
Table 1. Compressive property of PCL/TCP, PTMC/TCP, and 3.5. DOX release property
PTMC/PCL/TCP scaffolds
All scaffolds demonstrated substantial release rates and
Scaffolds Compressive Fσ=50% (N) steady drug controlled release properties during 130 days of
modulus (MPa) measurement (Figure 8). PTMC/TCP scaffolds with different
PCL 111.8 1157.7 TCP content showed similar DOX release rates. Compared
PCL/5%TCP 207.0 2065.8 with PTMC/TCP scaffolds, PTMC/PCL/TCP scaffolds
PCL/10%TCP 248.2 2223.0 displayed faster DOX release rates, presumably due to the
PCL/15%TCP 274.1 2423.8 large pores of scaffolds and high drug diffusion coefficient.
PCL/20%TCP 290.9 2570.1 PCL/TCP scaffolds displayed obviously slower release rates
PCL/25%TCP 298.8 2682.9 because of its low hydrophilicity, slow degradation rate, and
PTMC 1.5 81.4 low drug diffusion coefficient. Meanwhile, the DOX release
PTMC/5%TCP 5.6 312.8 rates of scaffolds increased with the increased of TCP content.
PTMC/10%TCP 7.1 371.5 PTMC/PCL/25%TCP and PTMC/25%TCP displayed 18.1%
PTMC/15%TCP 8.3 241.3 and 14.1% of cumulative DOX release, respectively, which
PTMC/20%TCP 10.1 303.1 were higher than PCL/TCP (3.1%). Thus, TCP improved
PTMC/25%TCP 13.5 470.6 the hydrophilicity, drug diffusion coefficients, and DOX
PTMC/PCL 76.5 1099.6 release rates of scaffolds. These results are consistent with
PTMC/PCL/5%TCP 86.2 1277.1 the micrographs structures of PCL/TCP, PTMC/TCP, and
PTMC/PCL/10%TCP 93.6 1299.2 PTMC/PCL/TCP scaffolds in this study.
PTMC/PCL/15%TCP 113.0 1348.3
PTMC/PCL/20%TCP 129.8 1468.6 3.6. In vitro biocompatibility assay
PTMC/PCL/25%TCP 154.8 1552.4
PCL: Poly(ε‑caprolactone); TCP: β‑tricalcium phosphate; PTMC: Poly (trimethyl Porous 3D-printed PCL/TCP, PTMC/TCP, and PTMC/
carbonate) PCL/TCP scaffolds can provide a large surface area, which
Volume 9 Issue 1 (2023) 280 https://doi.org/10.18063/ijb.v9i1.641