Page 300 - IJB-9-1
P. 300
International Journal of Bioprinting 3D printable conductive composite inks for biocompatible electrodes
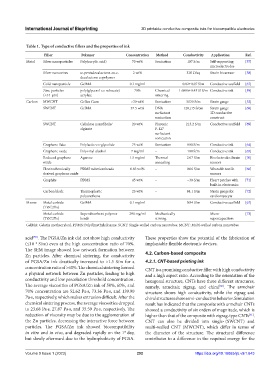
Table 1. Type of conductive fillers and the properties of ink
Filler Polymer Concentration Method Conductivity Application Ref.
Metal Silver nanoparticles Poly(acrylic acid) 70 wt% Sonication 107 S/m Self-supporting [57]
microelectrodes
Silver nanowires ω-pentadecalactone-co-ε- 2 wt% – 320 Ω/sq Strain biosensor [58]
decalactone copolymer
Gold nanoparticle GelMA 0.1 mg/ml – 0.82±0.07 S/m Conductive scaffold [67]
Zinc particles poly(glycerol-co-sebacate) 70% Chemical 1.6886±0.4310 S/m Conductive ink [59]
(<44 μm) acrylate sintering
Carbon MWCNT Gellan Gum >30 wt% Sonication 5030 S/m Strain gauge [52]
SWCNT GelMA 37.5 wt% DNA 128±15 S/cm Strain gauge [68]
surfactant 3D conductive
sonication construct
SWCNT Cellulose nanofibrils/ 20 wt% Pluronic 213.2 S/m Conductive scaffold [69]
alginate F-127
surfactant
sonication
Graphene flake Polylactic-co-glycolide 75 wt% Sonication 800 S/m Conductive ink [64]
Graphene oxide Polyvinyl alcohol 7 mg/ml – 100 S/m Conductive ink [65]
Reduced graphene Agarose 1.5 mg/ml Thermal 2.07 S/m Bioelectrodes Strain [70]
oxide annealing sensors
Electrochemically PDMS submicrobeads 0.83 vol% – 0.06 S/m Wearable tactile [66]
derived graphene oxide sensors
Graphite PDMS 45 wt% – ~30 S/m Heart patches with [71]
built-in electronics
Carbon black Thermoplastic 25 wt% – 84.1 S/m Strain gauge for [72]
polyurethane cardiomyocyte
Mxene Metal carbide GelMA 0.1 mg/ml – 0.94 S/m Conductive scaffold [67]
(Ti3C2Tx)
Metal carbide Superabsorbent polymer 290 mg/ml Mechanically – Micro- [73]
(Ti3C2Tx) beads stirring supercapacitors
GelMA: Gelatin methacryloyl, PDMS: Polydimethylsiloxane, SCNT: Single-walled carbon nanotubes, MCNT: Multi-walled carbon nanotubes
acid . The PGSA/Zn ink did not show high conductivity These properties show the potential of the fabrication of
[59]
−2
(≤10 S/m) even at the high concentration ratio of 70%. implantable flexible electronic devices.
The SEM image showed low network formation between
Zn particles. After chemical sintering, the conductivity 4.2. Carbon-based composite
of PGSA/Zn ink drastically increased to >1.5 S/m for a 4.2.1. CNT-based printing ink
concentration ratio of >60%. The chemical sintering formed CNT is a promising conductive filler with high conductivity
a physical network between Zn particles, leading to high and a high aspect ratio. According to the orientation of the
conductivity and low percolation threshold concentration. hexagonal structure, CNTs have three different structures,
The average viscosities of PGSA/Zn ink of 50%, 60%, and namely, armchair, zigzag, and chiral . The armchair
[60]
70% concentration are 52.62 Pa∙s, 73.36 Pa∙s, and 139.90 structure shows high conductivity, while the zigzag and
Pa∙s, respectively, which makes extrusion difficult. After the chiral structures show semi-conductive behavior. Simulation
chemical sintering process, the average viscosities dropped result has indicated that the composite with armchair CNTs
to 23.68 Pa∙s, 27.87 Pa∙s, and 35.59 Pa∙s, respectively. The showed a conductivity of six orders of magnitude, which is
reduction of viscosity may be due to the agglomeration of higher than that of the composite with zigzag-type CNTs .
[61]
the Zn particles, decreasing the interactive force between CNT can also be divided into single- (SWCNT) and
particles. The PGSA/Zn ink showed biocompatibility multi-walled CNT (MWCNT), which differ in terms of
in vitro and in vivo, and degraded rapidly on the 1 day, the diameter of the structure. The structural difference
st
but slowly afterward due to the hydrophobicity of PGSA. contributes to a difference in the required energy for the
Volume 9 Issue 1 (2023) 292 https://doi.org/10.18063/ijb.v9i1.643