Page 442 - IJB-9-5
P. 442
International Journal of Bioprinting Core-shell bioarchitectures
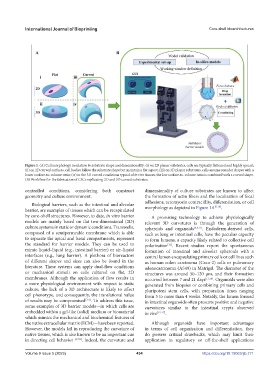
Figure 1. (A) Cell morphology in relation to substrate shape and dimensionality: (i) on 2D planar substrates, cells are typically flattened and highly spread;
(ii) on 2D curved surfaces, cell bodies follow the substrate shape but maintain a flat aspect; (iii) on 3D planar substrates, cells assume rounder shapes with a
lower surface-to-volume ratio; (iv) in the 3D curved conditions typical of in vivo tissues, the low surface-to-volume ratio is combined with a curved shape.
(B) Workflow for the fabrication of CSCs replicating 2D and 3D curved substrates.
controlled conditions, considering both construct dimensionality of culture substrates are known to affect
geometry and culture environment. the formation of actin fibers and the localization of focal
adhesions, actomyosin contractility, differentiation, or cell
Biological barriers, such as the intestinal and alveolar [9,10]
barrier, are examples of tissues which can be recapitulated morphology as depicted in Figure 1A .
by core–shell structures. However, to date, in vitro barrier A promising technology to achieve physiologically
models are mainly based on flat two-dimensional (2D) relevant 3D curvatures is through the generation of
culture systems in static or dynamic conditions. Transwells, spheroids and organoids [6,8,11] . Endoderm-derived cells,
composed of a semipermeable membrane which is able such as lung or intestinal cells, have the peculiar capacity
to separate the apical and basal compartments, represent to form lumens, a capacity likely related to collective cell
the standard for barrier models. They can be used to polarization . Recent studies report the spontaneous
[12]
mimic liquid–liquid (e.g., intestinal barrier) or air–liquid formation of intestinal and alveolar spheroids with a
interfaces (e.g., lung barrier). A plethora of bioreactors central lumen encapsulating primary cells or cell lines such
of different shapes and sizes can also be found in the as human colon carcinoma (Caco-2) cells or pulmonary
literature. These systems can apply dual-flow conditions adenocarcinoma (A549) in Matrigel. The diameter of the
or mechanical stimuli on cells cultured on flat, 2D structures was around 50–120 μm, and their formation
membranes. Although the application of flow results in occurred between 7 and 21 days [13,14] . Organoids were also
a more physiological environment with respect to static generated from biopsies or combining primary cells and
culture, the lack of a 3D architecture is likely to affect pluripotent stem cells, with preparation times ranging
cell phenotype, and consequently, the translational value from 3 to more than 4 weeks. Notably, the lumen formed
of results may be compromised [3-6] . To address this issue, in intestinal organoids often presents positive and negative
some examples of 3D barrier models—in which cells are curvatures similar to the intestinal crypts observed
embedded within a gel-like (solid) medium or biomaterial in vivo [15-17] .
which mimics the mechanical and biochemical features of
the native extracellular matrix (ECM)—have been reported. Although organoids have important advantages
However, the models fail in reproducing the curvature of in terms of cell organization and differentiation, they
native tissues, which is also known to be an important cue do possess critical drawbacks, which may limit their
in directing cell behavior [3,7,8] . Indeed, the curvature and application in regulatory or off-the-shelf applications
Volume 9 Issue 5 (2023) 434 https://doi.org/10.18063/ijb.771