Page 98 - ITPS-8-2
P. 98
INNOSC Theranostics and
Pharmacological Sciences Transformative natural product-drug combinations
Table 2. Bioactive phytoconstituents from Calotropis procera leaf extract combined with ampicillin. Reproduced with permission
from Gideon et al. 34
Pk# RT Area% Compound name Molecular weight
1 5.318 3.35 Cystamine 152.0
3 5.528 2.46 4-amino-1-Pentanol 103.0
5 5.959 1.42 N-methoxy-1-ribofuranosyl-4-imidazolecarboxylic amide 155.0
6 6.275 0.73 1,2,5-oxadiazol-3-carboxamide, 4,4’-azobis-, 2,2’-dioxide 284.0
9 7.361 1.94 3,3-dimethyl-4-(1-aminoethyl)-Azetidin-2-one 142.0
11 7.891 1.37 5-methyl-2-Heptanamine 128.0
20 12.065 1.36 dl-phenylephrine 167.0
23 14.598 1.16 Cystine 240.0
32 21.074 2.03 1,2,5-oxadiazol-3-carboxamide, 4,4’-azobis-, 2,2’-dioxide 284.0
34 22.127 0.73 Metaraminol 167.0
38 25.796 2.83 3-propoxyamphetamine 193.0
43 29.445 0.12 Hexadecanoic acid, methyl ester 270.0
49 30.437 2.68 1-docosene 308
50 31.083 0.16 9-octadecenoic acid (Z)-, methyl ester 296.0
52 36.087 0.78 3,7,11-trimethyl-2,6,10-dodecatrien-1-ol 222.0
53 36.620 13.04 Oleic acid 264.0
Abbreviations: Pk#: Number of peaks; RT: Retention time.
Table 3. Fourier transform infrared spectroscopy analysis of guava extract, aspirin, and the guava‑aspirin mixture, with
attributed functional groups. Reproduced with permission from Gideon 26
FTIR functional Wave number Guava extract Aspirin peaks (cm⁻¹) Reacted guava Associated phytochemicals
group region range (cm⁻¹) peaks (cm⁻¹) peaks (cm⁻¹)
O-H/N-H stretch 3,200 – 3,600 3,693.8; 3,280.1 3,488.8 3,235.3 Alcohols, phenols, amines, or
carboxylic acids
C-H stretch 2,850 – 3,000 2,918.5; 2,851.4 2,870.1; 2,959.5; 2,698.6; 2,918.5 Alkanes
2,586.8; 2,545.8
Clkanes stretch 2,100 – 2,260 2,109.7; 1,994.1 2,091.0 2,105.9 Alkynes or nitriles
C=O stretch 1,630 – 1,820 1,617.7 1,804.0; 1,837.6; 1,751.8; 1,748.1, 1,677.3, Ketones, aldehydes, esters, or
1,684.3; 1,602.8 1,602.8 carboxylic acids
N-H bending 1,500 – 1,600 - - - Primary and secondary amines
C-H bending 1,400 – 1,500 1449.9 1483.5; 1457.4 1453.7 Aromatic compounds
Abbreviation: FTIR: Fourier transform infrared spectroscopy.
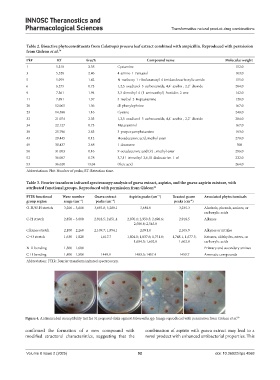
Figure 4. Antimicrobial susceptibility test for 31 prepared disks against Salmonella spp. Image reproduced with permission from Gideon et al. 39
confirmed the formation of a new compound with combination of aspirin with guava extract may lead to a
modified structural characteristics, suggesting that the novel product with enhanced antibacterial properties. This
Volume 8 Issue 2 (2025) 92 doi: 10.36922/itps.4068