Page 42 - JCBP-3-1
P. 42
Journal of Clinical and
Basic Psychosomatics Psychosomatic influences on insomnia
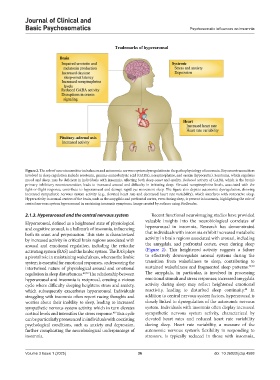
Figure 2. The role of neurotransmitter imbalances and autonomic nervous system dysregulation in the pathophysiology of insomnia. Key neurotransmitters
involved in sleep regulation include serotonin, gamma-aminobutyric acid (GABA), norepinephrine, and orexin (hypocretin). Serotonin, which regulates
mood and sleep, may be deficient in individuals with insomnia, affecting both sleep onset and quality. Reduced activity of GABA, which is the brain’s
primary inhibitory neurotransmitter, leads to increased arousal and difficulty in initiating sleep. Elevated norepinephrine levels, associated with the
fight-or-flight response, contribute to hyperarousal and disrupt rapid eye movement sleep. The figure also depicts autonomic dysregulation, showing
increased sympathetic nervous system activity (e.g., elevated heart rate and decreased heart rate variability), which interferes with restorative sleep.
Hyperactivity in arousal centers of the brain, such as the amygdala and prefrontal cortex, even during sleep, is present in insomnia, highlighting the role of
central nervous system hyperarousal in sustaining insomnia symptoms. Image created by authors using BioRender.
2.1.3. Hyperarousal and the central nervous system Recent functional neuroimaging studies have provided
Hyperarousal, defined as a heightened state of physiological valuable insights into the neurobiological correlates of
and cognitive arousal, is a hallmark of insomnia, influencing hyperarousal in insomnia. Research has demonstrated
both its onset and perpetuation. This state is characterized that individuals with insomnia exhibit increased metabolic
by increased activity in critical brain regions associated with activity in brain regions associated with arousal, including
arousal and emotional regulation, including the reticular the amygdala and prefrontal cortex, even during sleep
activating system (RAS) and the limbic system. The RAS plays (Figure 2). This heightened activity suggests a failure
a pivotal role in maintaining wakefulness, whereas the limbic to effectively downregulate arousal systems during the
system is essential for emotional responses, underscoring the transition from wakefulness to sleep, contributing to
intertwined nature of physiological arousal and emotional sustained wakefulness and fragmented sleep patterns. 49,50
regulation in sleep disturbances. 49,50 The relationship between The amygdala, in particular, is involved in processing
hyperarousal and insomnia is reciprocal, creating a vicious emotional stimuli and stress responses; increased amygdala
cycle where difficulty sleeping heightens stress and anxiety, activity during sleep may reflect heightened emotional
48
which subsequently exacerbates hyperarousal. Individuals reactivity, leading to disturbed sleep continuity. In
struggling with insomnia often report racing thoughts and addition to central nervous system factors, hyperarousal is
worries about their inability to sleep, leading to increased closely linked to dysregulation of the autonomic nervous
sympathetic nervous system activity, which in turn elevates system. Individuals with insomnia often display increased
48
cortisol levels and intensifies the stress response. This cycle sympathetic nervous system activity, characterized by
can be particularly pronounced in individuals with coexisting elevated heart rates and reduced heart rate variability
psychological conditions, such as anxiety and depression, during sleep. Heart rate variability, a measure of the
further complicating the neurobiological underpinnings of autonomic nervous system’s flexibility in responding to
insomnia. stressors, is typically reduced in those with insomnia,
Volume 3 Issue 1 (2025) 36 doi: 10.36922/jcbp.4588