Page 43 - JCTR-10-4
P. 43
Biberstein et al. | Journal of Clinical and Translational Research 2024; 10(4): 263-268 265
visualized under the baseplate and measured as the percentage potentially improve tibial implant fixation by incorporating
of the surface area of the tibial tray that was involved. Given cementation pockets [15-20]. While cement pockets increase
variable keel geometries and sizes between implants A and B, the surface area for fixation, their ability to improve fixation has
the area of the keel was subtracted from the area of the entire yet to be demonstrated. In addition, there has been an increased
baseplate before the calculation of percent contamination emphasis on improving implant-cement interface fixation as
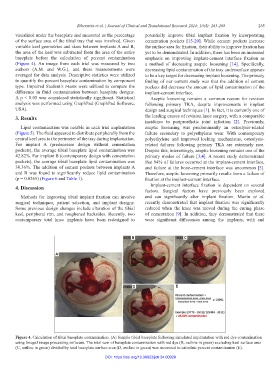
(Figure 4). An image from each trial was measured by two a method of decreasing aseptic loosening [14]. Specifically,
authors (A.M. and W.G.), and these measurements were decreasing lipid contamination of the tray undersurface appears
averaged for data analysis. Descriptive statistics were utilized to be a key target for decreasing implant loosening. The primary
to quantify the percent baseplate contamination by component finding of our current study was that the addition of cement
type. Unpaired Student’s t-tests were utilized to compare the pockets did decrease the amount of lipid contamination of the
difference in fluid contamination between baseplate designs. implant-cement interface.
A p < 0.05 was considered statistically significant. Statistical Aseptic loosening remains a common reason for revision
analysis was performed using GraphPad (GraphPad Software, following primary TKA, despite improvements in implant
USA). design and surgical techniques [1]. In fact, it is currently one of
3. Results the leading causes of revision knee surgery, with a comparable
incidence to periprosthetic joint infection [2]. Previously,
Lipid contamination was notable in each trial implantation aseptic loosening was predominantly an osteolysis-related
(Figure 5). The fluid appeared to distribute peripherally from the failure secondary to polyethylene wear. With contemporary
central keel area to the perimeter of the tray during implantation. polyethylene and improved locking mechanisms, osteolysis-
For implant A (predecessor design without cementation related failures following primary TKA are extremely rare.
pockets), the average tibial baseplate lipid contamination was Despite this, interestingly, aseptic loosening remains one of the
42.82%. For implant B (contemporary design with cementation primary modes of failure [3,4]. A recent study demonstrated
pockets), the average tibial baseplate lipid contamination was that 94% of failures occurred at the implant-cement interface,
30.36%. The addition of cement pockets between implants A and failure at the bone-cement interface was uncommon [5].
and B was found to significantly reduce lipid contamination Therefore, aseptic loosening primarily results from a failure of
(p = 0.0265) (Figure 6 and Table 1). fixation at the implant-cement interface.
4. Discussion Implant-cement interface fixation is dependent on several
factors. Surgical factors have previously been explored
Methods for improving tibial implant fixation can involve and can significantly alter implant fixation. Martin et al.
surgical techniques, patient selection, and implant designs. recently demonstrated that implant fixation was significantly
Some previous design changes include alteration of the tibial reduced when the knee was moved during the curing phase
keel, peripheral rim, and roughened backsides. Recently, two of cementation [9]. In addition, they demonstrated that there
contemporary total knee implants have been redesigned to were significant differences among the implants, with and
B C
A
D E
Figure 4. Calculation of tibial baseplate contamination. (A) Sample tibial baseplate following simulated implantation with red dye contamination
using ImageJ image processing software. The total sum of baseplate contamination with red dye (B, outline in green) excluding keel surface area
(C, outline in green) divided by total baseplate surface area (D, outline in green) was measured to calculate percent contamination (E).
DOI: https://doi.org/10.36922/jctr.24.00029