Page 42 - JCTR-10-4
P. 42
264 Biberstein et al. | Journal of Clinical and Translational Research 2024; 10(4): 263-268
is now accepted that both implant and surgical factors impact
fixation [5,7-9]. More specifically, these include component
malalignment, improper bone surface preparation, and drying,
poor cement technique including mixing and handling,
potentially high-viscosity cements, and smaller cement mantles,
as well as other intraoperative surgical technique errors. These
problems can all detrimentally affect the cement structure and
strength at the implant-cement interface, potentially increasing
the risk of component debonding and, subsequently, aseptic
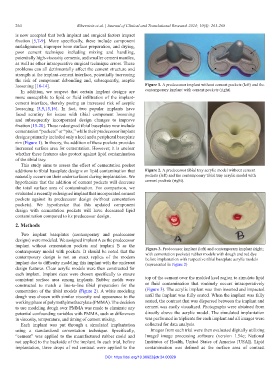
loosening [10-14]. Figure 1. A predecessor implant without cement pockets (left) and the
In addition, we suspect that certain implant designs are contemporary implant with cement pockets (right).
more susceptible to lipid or fluid infiltration of the implant-
cement interface, thereby posing an increased risk of aseptic
loosening [5,9,15,16]. In fact, two popular implants have
faced scrutiny for issues with tibial component loosening
and subsequently incorporated design changes to improve
fixation [15-20]. These redesigned tibial baseplates now include
cementation “pockets” or “pits,” while their predecessor implant
designs primarily included only a keel and a peripheral baseplate
rim (Figure 1). In theory, the addition of these pockets provides
increased surface area for cementation. However, it is unclear
whether these features also protect against lipid contamination
of the tibial tray.
This study aims to assess the effect of cementation pocket
additions to tibial baseplate designs on lipid contamination that Figure 2. A predecessor tibial tray acrylic model without cement
naturally occurs on their undersurfaces during implantation. We pockets (left) and the contemporary tibial tray acrylic model with
hypothesize that the addition of cement pockets will decrease cement pockets (right).
the total surface area of contamination. For comparison, we
evaluated a recently redesigned implant that incorporated cement
pockets against its predecessor design (without cementation
pockets). We hypothesize that this updated component
design with cementation pockets will have decreased lipid
contamination compared to its predecessor design.
2. Methods
Two implant baseplates (contemporary and predecessor
designs) were modeled. We assigned implant A as the predecessor
implant without cementation pockets and implant B as the Figure 3. Predecessor implant (left) and contemporary implant (right;
contemporary model with pockets. It should be noted that the with cementation pockets) rubber models with dough and red dye
contemporary design is not an exact replica of the modern before implantation with respective tibial baseplate acrylic models
implant due to difficulty modeling this implant with the undercut (represented in Figure 2)
design features. Clear acrylic models were then constructed for
each implant. Implant sizes were chosen specifically to ensure
consistent surface area among implants. Rubber molds were top of the cement over the molded keel region to simulate lipid
constructed to match a line-to-line tibial preparation for the or fluid contamination that routinely occurs intraoperatively
cementation of the tibial models (Figure 2). A white modeling (Figure 3). The acrylic implant was then inserted and impacted
dough was chosen with similar viscosity and appearance to the until the implant was fully seated. When the implant was fully
working phase of polymethylmethacrylate (PMMA). The decision seated, the contrast that was dispersed between the implant and
to use modeling dough over PMMA was made to eliminate any cement was easily visualized. Photographs were obtained from
potential confounding variables with PMMA, such as differences directly above the acrylic model. The simulated implantation
in viscosity, temperature, and timing of cement mixing. was performed in triplicate for each implant and all images were
Each implant was put through a simulated implantation collected for data analysis.
using a standardized cementation technique. Specifically, Images from each trial were then evaluated digitally utilizing
“cement” was applied to the manufactured rubber mold and ImageJ image processing software (version 1.54e; National
not applied to the backside of the implant. In each trial, before Institutes of Health, United States of America [USA]). Lipid
implantation, three drops of red contrast were applied to the contamination was defined as the surface area of contrast
DOI: https://doi.org/10.36922/jctr.24.00029