Page 13 - JCTR-11-1
P. 13
Journal of Clinical and
Translational Research Cannabinoids for cannabis use disorder
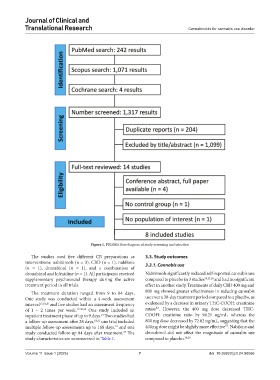
Figure 1. PRISMA flow diagram of study screening and selection
The studies used five different CB preparations as 3.3. Study outcomes
interventions: nabiximols (n = 3), CBD (n = 1), nabilone 3.3.1. Cannabis use
(n = 1), dronabinol (n = 1), and a combination of
dronabinol and lofexidine (n = 1). All participants received Nabiximols significantly reduced self-reported cannabis use
supplementary psychosocial therapy during the active compared to placebo in 3 studies 19,23,24 and had no significant
treatment period in all trials. effect in another study. Treatments of daily CBD 400 mg and
The treatment duration ranged from 9 to 84 days. 800 mg showed greater effectiveness in reducing cannabis
One study was conducted within a 4-week assessment use over a 30-day treatment period compared to a placebo, as
interval 17,18,20 and five studies had an assessment frequency evidenced by a decrease in urinary THC-COOH: creatinine
of 1 – 2 times per week. 17-20,22 One study included an ratios²². However, the 400 mg dose decreased THC-
21
inpatient treatment phase of up to 9 days. Two studies had COOH: creatinine ratio by 94.21 ng/mL, whereas the
a follow-up assessment after 28 days, 18,21 one trial included 800 mg dose decreased by 72.02 ng/mL, suggesting that the
multiple follow-up assessments up to 168 days, and one 400mg dose might be slightly more effective²². Nabilone and
22
study conducted follow-up 84 days after treatment. The dronabinol did not affect the magnitude of cannabis use
24
study characteristics are summarized in Table 1. compared to placebo. 18,20
Volume 11 Issue 1 (2025) 7 doi: 10.36922/jctr.24.00066