Page 15 - JCTR-11-1
P. 15
Journal of Clinical and
Translational Research Cannabinoids for cannabis use disorder
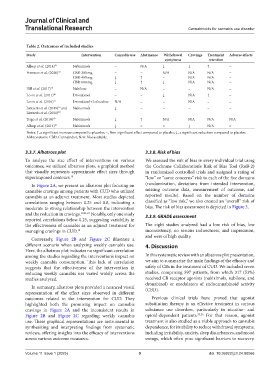
Table 2. Outcomes of included studies
Study Intervention Cannabis use Abstinence Withdrawal Cravings Treatment Adverse effects
symptoms retention
Allsop et al. (2014) 21 Nabiximols = N/A ↓ ↓ ↑ =
Freeman et al. (2020) 22 CBD 200 mg = = N/A N/A N/A =
CBD 400 mg ↓ ↑ = N/A N/A =
CBD 800 mg ↓ ↑ ↓ N/A N/A =
Hill et al. (2017) 18 Nabilone = N/A = = N/A =
Levin et al. (2011) 20 Dronabinol = = ↓ N/A ↑ =
Levin et al. (2016) 17 Dronabinol+Lofexidine N/A = = N/A = =
Lintzeris et al. (2019) and Nabiximols ↓ = = = = =
23
Lintzeris et al. (2020) 24
Trigo et al. (2018) 19 Nabiximols ↓ ↑ N/A N/A N/A N/A
Allsop et al. (2014) 21 Nabiximols ↓ = = ↓ N/A =
Notes: ↑, a significant increase compared to placebo; =, Non-significant effect compared to placebo; ↓, a significant reduction compared to placebo.
Abbreviations: CBD: Cannabidiol; N/A: Not available.
3.3.7. Albatross plot 3.3.8. Risk of bias
To analyze the size effect of interventions on various We assessed the risk of bias in every individual trial using
outcomes, we utilized albatross plots, a graphical method the Cochrane Collaboration’s Risk of Bias Tool (RoB-2)
that visually represents approximate effect sizes through in randomized controlled trials and assigned a rating of
superimposed contours. 15 “low” or “some concerns” risk to each of the five domains
In Figure 2A, we present an albatross plot focusing on (randomization, deviations from intended intervention,
cannabis cravings among patients with CUD who utilized missing outcome data, measurement of outcome, and
cannabis as an adjunct treatment. Most studies depicted reported results). Based on the number of domains
correlations ranging between 0.25 and 0.8, indicating a classified as “low risk,” we also created an “overall” risk of
moderate to strong relationship between the intervention bias. The risk of bias assessment is depicted in Figure 3.
and the reduction in cravings. 19,21,23 Notably, only one study 3.3.9. GRADE assessment
reported correlations below 0.25, suggesting variability in
the effectiveness of cannabis as an adjunct treatment for The eight studies analyzed had a low risk of bias, low
managing cravings in CUD. 18 inconsistency, no serious indirectness, and imprecision,
and were of high quality.
Conversely, Figure 2B and Figure 2C illustrate a
different scenario when analyzing weekly cannabis use. 4. Discussion
Here, the albatross plot indicates no significant correlation
among the studies regarding the intervention’s impact on In this systematic review with an albatross plot presentation,
weekly cannabis consumption. This lack of correlation we aim to summarize the main findings of the efficacy and
suggests that the effectiveness of the intervention in safety of CBs in the treatment of CUD. We included seven
reducing weekly cannabis use varied widely across the studies, comprising 597 patients, from which 317 (53%)
studies analyzed. received CB receptor agonists (nabiximols, nabilone, and
dronabinol) or modulators of endocannabinoid activity
In summary, albatross plots provided a nuanced visual (CBD).
representation of the effect sizes observed in different
outcomes related to the intervention for CUD. They Previous clinical trials have proved that agonist
highlighted both the promising impact on cannabis substitution therapy is an effective treatment in various
cravings in Figure 2A and the inconsistent results in substance use disorders, particularly in nicotine- and
Figure 2B and Figure 2C regarding weekly cannabis opioid-dependent patients. 25,26 For that reason, agonist
use. These graphical representations are instrumental in treatment is also studied as a viable approach to cannabis
synthesizing and interpreting findings from systematic dependence, for its ability to reduce withdrawal symptoms,
reviews, offering insights into the efficacy of interventions including irritability, anxiety, sleep disturbances, and mood
across various outcome measures. swings, which often pose significant barriers to recovery.
Volume 11 Issue 1 (2025) 9 doi: 10.36922/jctr.24.00066