Page 14 - JCTR-11-1
P. 14
Journal of Clinical and
Translational Research Cannabinoids for cannabis use disorder
3.3.2. Abstinence
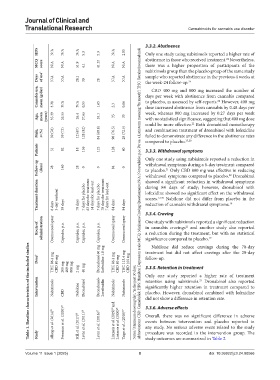
HDS N/A N/A N/A 5.3 5.3 N/A 2.85 Only one study using nabiximols reported a higher rate of
24
MCQ score N/A N/A 51.9 4.1 41.25 N/A N/A abstinence in those who received treatment. Nevertheless,
there was a higher proportion of participants of the
nabiximols group than the placebo group of the same study
Days of use † N/A N/A 28.1 30 28 N/A N/A sample who reported abstinence in the previous 4 weeks at
24
the week-24 follow-up.
Cannabis use, mean (g/day) 3.28 N/A N/A 0.55 1.65 2.3 0.86 days per week with abstinence from cannabis compared
CBD 400 mg and 800 mg increased the number of
to placebo, as assessed by self-reports. However, 400 mg
22
week, whereas 800 mg increased by 0.27 days per week
Age, mean (years) 35.39 26.55 26.4 37.65 35.1 35 33 dose increased abstinence from cannabis by 0.48 days per
with no statistical significance, suggesting that 400 mg dose
could be more effective. Both dronabinol monotherapy
22
Male, n (%) 39 (76) 59 (72) 12 (67) 128 (82) 84 (68.8) 98 (76.5) 29 (72.5) and combination treatment of dronabinol with lofexidine
failed to demonstrate any difference in the abstinence rates
compared to placebo. 17,20
Patients 51 82 18 156 122 128 40 3.3.3. Withdrawal symptoms
Only one study using nabiximols reported a reduction in
Follow‑up (days) 28 140 28 0 0 84 0 withdrawal symptoms during a 6-day treatment compared
to placebo. Only CBD 800 mg was effective in reducing
21
withdrawal symptoms compared to placebo. Dronabinol
22
showed a significant reduction in withdrawal symptoms
Treatment duration 3-day washout 7 days for placebo 63 days for treatment 14 days for lead-out 7 days for placebo 70 days for treatment 7 days for lead-out Abbreviations: CBD: Cannabidiol; HDS: Hamilton Depression scale; MCQ: Marijuana Craving Questionnaire; N/A: Not available; p.o.: Per os, a Latin term meaning “by mouth”; THC: Tetrahydrocannabinol. during 84 days of study; however, dronabinol with
lofexidine showed no significant effect on the withdrawal
Nabilone did not differ from placebo in the
scores.
17,20
30 days
70 days
84 days
84 days
6 days
reduction of cannabis withdrawal symptoms.
18
3.3.4. Craving
Route of administration Oromucosal spray Capsules, p.o. Capsules, p.o. Capsules, p.o. Capsules, p.o. Oromucosal spray Oromucosal spray One study with nabiximols reported a significant reduction
in cannabis cravings and another study also reported
21
a reduction during the treatment, but with no statistical
significance compared to placebo.
19
Table 1. Baseline characteristics of the included studies
Nabilone did reduce cravings during the 70-day
THC 86.4 mg CBD 80 mg 200 mg 400 mg 800 mg 2 mg 40 mg THC 86.4 mg CBD 80 mg THC 113.4 mg CBD 105 mg 3.3.5. Retention in treatment
Dose § Dronabinol 60 mg Lofexidine 1.8 mg treatment but did not affect cravings after the 28-day
follow-up.
Intervention Nabiximols CBD Nabilone Dronabinol Dronabinol+ Lorefexidin Nabiximols Nabiximols Notes: § Maximum dose/mg/day; † in the past 30 days. Only one study reported a higher rate of treatment
retention using nabiximols. Dronabinol also reported
21
significantly higher retention in treatment compared to
placebo. However, dronabinol combined with lofexidine
did not show a difference in retention rate.
Allsop et al. (2014) 21 Freeman et al. (2020) 22 Hill et al. (2017) 18 Levin et al. (2011) 20 Levin et al. (2016) 17 Lintzeris et al. (2019) 23 and Lintzeris et al. (2020) 24 Trigo et al., (2018) 19 3.3.6. Adverse effects
Overall, there was no significant difference in adverse
events between intervention and placebo reported in
any study. No serious adverse event related to the study
Study procedure was recorded in the intervention group. The
study outcomes are summarized in Table 2.
Volume 11 Issue 1 (2025) 8 doi: 10.36922/jctr.24.00066