Page 71 - JCTR-11-3
P. 71
Journal of Clinical and
Translational Research N-NOSE: Early cervical cancer screening
A B
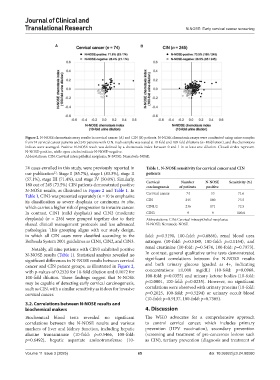
Figure 2. N-NOSE chemotaxis assay results in cervical cancer (A) and CIN (B) patients. N-NOSE chemotaxis assays were conducted using urine samples
from 74 cervical cancer patients and 245 patients with CIN. Each sample was tested at 10-fold and 100-fold dilutions (n=10/dilution), and the chemotaxis
indices were averaged. Positive N-NOSE result was defined by a chemotaxis index between 0 and 1 in at least one dilution. Closed circles represent
N-NOSE-positive, while open circles indicate N-NOSE-negative.
Abbreviations: CIN: Cervical intraepithelial neoplasia; N-NOSE: Nematode-NOSE.
74 cases enrolled in this study, were previously reported in Table 1. N-NOSE sensitivity for cervical cancer and CIN
our publication : Stage 0 (85.7%), stage I (83.3%), stage II patients
23
(57.1%), stage III (71.4%), and stage IV (50.0%). Similarly, Cervical Number N-NOSE Sensitivity (%)
180 out of 245 (73.5%) CIN patients demonstrated positive carcinogenesis of patients positive
N-NOSE results, as illustrated in Figure 2 and Table 1. In Cervical cancer 74 53 71.6
Table 1, CIN3 was presented separately (n = 9) to emphasize
its classification as severe dysplasia or carcinoma in situ, CIN 245 180 73.5
which carries a higher risk of progression to invasive cancer. CIN1/2 236 171 72.5
In contrast, CIN1 (mild dysplasia) and CIN2 (moderate CIN3 9 9 100.0
dysplasia) (n = 236) were grouped together due to their Abbreviations: CIN: Cervical intraepithelial neoplasia;
shared clinical management protocols and less advanced N-NOSE: Nematode-NOSE.
pathologies. This grouping aligns with our study design,
in which all CIN cases were classified according to the fold: p=0.3190, 100-fold: p=0.6866), renal blood urea
Bethesda System 2001 guidelines as CIN1, CIN2, and CIN3. nitrogen (10-fold: p=0.5180, 100-fold: p=0.1154), and
Notably, all nine patients with CIN3 exhibited positive renal creatinine (10-fold: p=0.5474, 100-fold: p=0.7973).
N-NOSE results (Table 1). Statistical analysis revealed no In contrast, general qualitative urine tests demonstrated
significant differences in N-NOSE results between cervical significant correlations between the N-NOSE results
cancer and CIN patient groups, as illustrated in Figure 2, and both urinary glucose (graded as 4+, indicating
with p-values of 0.2520 for 10-fold dilution and 0.1072 for concentrations ≥1,000 mg/dL) (10-fold: p=0.0968,
100-fold dilution. These findings suggest that N-NOSE 100-fold: p=0.0355) and urinary ketone bodies (10-fold:
may be capable of detecting early cervical carcinogenesis, p<0.0001, 100-fold: p=0.0235). However, no significant
such as CIN, with a similar sensitivity as it does for invasive correlations were observed with urinary proteins (10-fold:
cervical cancer. p=0.2025, 100-fold: p=0.5294) or urinary occult blood
(10-fold: p=0.9137, 100-fold: p=0.7385).
3.2. Correlations between N-NOSE results and
biochemical makers 4. Discussion
Biochemical blood tests revealed no significant The WHO advocates for a comprehensive approach
correlations between the N-NOSE results and various to control cervical cancer, which includes primary
markers of liver and kidney function, including hepatic prevention (HPV vaccination), secondary prevention
alanine transaminase (10-fold: p=0.5466, 100-fold: (screening and treatment of pre-cancerous lesions such
p=0.6492), hepatic aspartate aminotransferase (10- as CIN), tertiary prevention (diagnosis and treatment of
Volume 11 Issue 3 (2025) 65 doi: 10.36922/jctr.24.00080