Page 15 - JCTR-9-4
P. 15
AboEl-Azm et al. | Journal of Clinical and Translational Research 2023; 9(4): 222-235 231
A
B
C
D
E
F
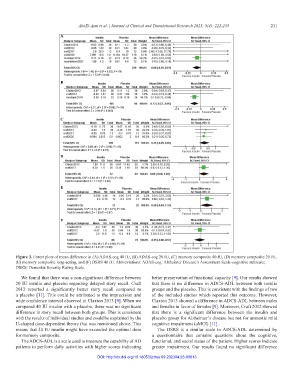
Figure 3. Forest plots of mean difference in (A) ADAS-cog 40 IU, (B) ADAS-cog 20 IU, (C) memory composite 40 IU, (D) memory composite 20 IU,
(E) memory composite long-acting, and (F) DSRS 40 IU. Abbreviations: ADAS-cog: Alzheimer Disease’s Assessment Scale-cognitive subscale;
DSRS: Dementia Severity Rating Scale.
We found that there was a non-significant difference between better preservation of functional capacity [9]. Our results showed
20 IU insulin and placebo regarding delayed story recall. Craft that there is no difference in ADCS-ADL between both insulin
2012 reported a significantly better story recall compared to groups and the placebo. This is consistent with the findings of two
a placebo [11]. This could be attributed to the imprecision and of the included studies which reported this outcome. However,
wide confidence interval observed in Claxton 2013 [9]. When we Claxton 2013 showed a difference in ADCS-ADL between males
compared 40 IU insulin with a placebo, there was no significant and females in favor of females [9]. Moreover, Craft 2012 showed
difference in story recall between both groups. This is consistent that there is a significant difference between the insulin and
with the results of individual studies and could be explained by the placebo group for Alzheimer’s disease but not for amnestic mild
U-shaped dose-dependent theory that was mentioned above. This cognitive impairment (aMCI) [11].
means that 40 IU insulin might have exceeded the optimal dose The DSRS is a similar scale to ADCS-ADL determined by
for memory composite. a questionnaire that contains questions about the cognitive,
The ADCS-ADL is a scale used to measure the capability of AD functional, and social status of the patient. Higher scores indicate
patients to perform daily activities with higher scores indicating greater impairment. Our results found no significant difference
DOI: http://dx.doi.org/10.18053/jctres.09.202304.23-00013