Page 14 - JCTR-9-4
P. 14
230 AboEl-Azm et al. | Journal of Clinical and Translational Research 2023; 9(4): 222-235
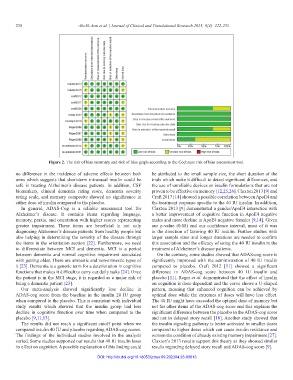
Figure 2. The risk of bias summary and risk of bias graph according to the Cochrane risk of bias assessment tool.
no difference in the incidence of adverse effects between both be attributed to the small sample size, the short duration of the
arms which suggests that short-term intranasal insulin could be trials which make it difficult to detect significant differences, and
safe in treating Alzheimer’s disease patients. In addition, CSF the use of unreliable devices or insulin formulations that are not
biomarkers, clinical dementia rating score, dementia severity proven to be effective on memory [12,25,26]. Claxton 2013 [9] and
rating scale, and memory composite showed no significance in Craft 2017 [14] showed a possible correlation between ApoE4 and
either dose of insulin compared to the placebo. the treatment response specific to the 40 IU insulin. In addition,
In general, ADAS-Cog is a reliable assessment tool for Claxton 2013 [9] demonstrated a gender/ApoE4 interaction with
Alzheimer’s disease. It contains items regarding language, a better improvement of cognitive function in ApoE4 negative
memory, praxis, and orientation with higher scores representing males and more decline in ApoE4 negative females [9,14]. Given
greater impairment. These items are beneficial in not only our p-value (0.08) and our confidence interval, most of it was
diagnosing Alzheimer’s disease patients from healthy people but in the direction of favoring 40 IU insulin. Further studies with
also helping in determining the severity of the disease through larger sample sizes and longer durations are needed to confirm
the items in the orientation section [22]. Furthermore, we need this association and the efficacy of using the 40 IU insulin in the
to differentiate between MCI and dementia. MCI is a period treatment of Alzheimer’s disease patients.
between dementia and normal cognitive impairment associated On the contrary, some studies showed that ADAS-cog score is
with getting older. There are amnestic and non-amnestic types of significantly improved with the administration of 40 IU insulin
it [23]. Dementia is a generic term for a deterioration in cognitive compared to placebo. Craft 2012 [11] showed a significant
functions that makes it difficult to carry out daily tasks [24]. Once difference in ADAS-cog score between 40 IU insulin and
the patient is in the MCI stage, it is regarded as a major risk of placebo [11]. Reger et al. demonstrated that the effect of insulin
being a dementia patient [23]. on cognition is dose-dependent and the curve shows a U-shaped
Our meta-analysis showed significantly less decline in pattern, meaning that enhanced cognition can be achieved by
ADAS-cog score from the baseline in the insulin 20 IU group optimal dose while the extremes of doses will have less effect.
when compared to the placebo. This is consistent with individual The 40 IU might have exceeded the optimal dose of memory but
study results which showed that the insulin group had less not for other items of the ADAS-cog score and this explains the
decline in cognitive function over time when compared to the significant difference between the placebo in the ADAS-cog score
placebo [9,11,13]. and not in delayed story recall [18]. Another study showed that
The results did not reach a significant cutoff point when we the insulin signaling pathway is better activated in smaller doses
compared insulin 40 IU and placebo regarding ADAS-cog scores. compared to higher doses which can cause insulin resistance and
The findings of the individual studies involved in the analysis worsen the condition of already existing memory impairment [27].
varied. Some studies supported our results that 40 IU insulin loses Claxton’s 2013 results support this theory as they showed similar
its effect on cognition. A possible explanation of this finding could results regarding delayed story recall and ADAS-cog score [9].
DOI: http://dx.doi.org/10.18053/jctres.09.202304.23-00013